VARSITY – Vedolizumab versus Adalimumab for UC
Vedolizumab versus Adalimumab for Moderate-to-Severe Ulcerative Colitis.
Sands BE, Peyrin-Biroulet L, Loftus EV Jr, et al.VARSITY Study Group.
N Engl J Med. 2019 Sep 26;381(13):1215-1226. [Full text]
Summary by Esha Parikh
Consensus guidelines from 2017 recommended biologic agents for patients with moderate-to-severe ulcerative colitis (UC) who have not responded to conventional therapies such as aminosalicylates, corticosteroids, or other immunomodulators [1]. Historically, treatment biologic options for UC have been TNF alpha inhibitors; however, a host of new agents including vedolizumab, ustekinumab and tofacitinb are now available for treatment of moderate to severe ulcerative colitis.
Vedolizumab is an ‘integrin antagonist’ that binds to the α4β7 integrin on the surface of T-lymphocytes. The α4β7 integrin on the T-cell allows binding to a mucosal addressin cell adhesion molecule-1 (MAdCAM-1) on GI endothelial cells, allowing for migration to the mucosal layers
Until recently, each biologic for UC had been compared to placebo, but there was a lack of studies comparing one biologic to another. The VARSITY trial was the first head-to-head randomized controlled trial comparing vedolizumab (Entyvio) to adalimumab (Humira). More recently another trial, SEAVUE, directly compared ustekinumab with adalimumab in moderate to severe Crohn’s disease [2].
Patient population and Design
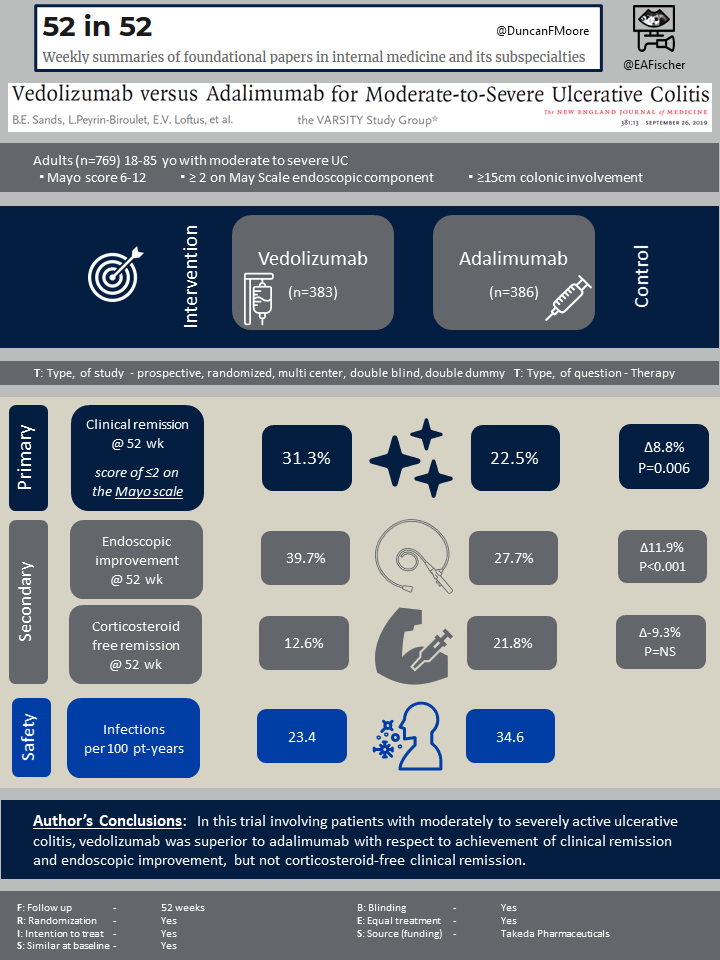
A total of 769 patients underwent randomization (383 receiving vedolizumab and 386 receiving adalimumab). Inclusion criteria for those eligible for the trial included adults aged 18 to 85 with moderate to severe active UC, with a total Mayo score of 6-12 and a subscore of at least 2 on the endoscopic component of the May Scale, at least 15cm of colonic involvement, and a confirmed diagnosis of UC prior to screening. Patients who had not previously used a TNF inhibitor and had no response to conventional medical therapy were also eligible. Patients who were previously on a TNF inhibitor (besides adalimumab) who had discontinued treatment for other reasons beside safety were also eligible but could only make up 25% of the total patient population.
Outcomes
The primary outcome studied was clinical remission defined as a total score of ≤2 on the Mayo scale and no subscore >1 on any of the score’s four components. Secondary outcomes including endoscopic improvement (defined as a subscore of 0 or 1 on the Mayo endoscopic component) and corticosteroid free clinical remission for patients receiving steroids at baseline.
Other additional endpoints that were studied included durable clinical remission (remission at weeks 14 and 52), improvements in subscores on patient-reported components of the Mayo scale, improvements in quality of life, histologic remission, minimal histologic disease, clinical response, and safety. The overall outcomes show that vedolizumab was superior to adalimumab in achieving clinical remission and endoscopic improvement, both statistically significant, but was not superior in corticosteroid-free clinical remission, though not statistically significant.
Results
Clinical remission at week 52 (primary outcome) was more frequent in the vedolizumab group (31.3%) than in the adalimumab group (22.5%), Δ8.8%, P=0.006. Endoscopic improvement at week 52 (secondary outcome) was also observed more frequently in the vedolizumab group (39.7%) than in the adalimumab group (27.7%) Δ11.9%, P<0.001. Yet corticosteroid-free clinical remission at 52 weeks (another secondary outcome) favored adalimumab, but this was not statistically significant. It was observed in less patients in the vedolizumab group 12.6% than in the adalimumab group 21.8%, Δ−9.3%. There were other secondary outcomes that favored vedolizumab , namely those with a a subscore of 0 on both the rectal bleeding and endoscopic components of the Mayo scale and tose with a subscore of 0 or 1 on the stool frequency component.
Any and serious adverse events occurred frequently with both vedolizumab and adalimumab group (Table 2). Infections seemed to occur less frequently with vedolizumab than with adalimumab (23.4 vs. 34.6 events per 100 patient-years; serious infections, 1.6 vs. 2.2 events per 100 patient-years). Specifically herpes zoster was less frequent with vedolizumab than with adalimumab (0.5 vs. 4.2 per 100 patient-years), but interestingly C. difficile infection was more frequent (1.1 vs. 0.6 per 100 patient-years).
Discussion
As noted, the mainstay of inducing remission in moderate to severe UC have been TNF inhibitors, including adalimumab; however, data from the VARSITY trial does seem to suggest improved outcomes with vedolizumab, though there may be more corticosteroid need in that population. The SEAVUE trial interestingly did not show a significant difference when comparing ustekinumab versus adalimumab for inducing remission in severe Crohn’s disease[2]. Ustekinumab’s mechanism is distinct from vedolizumab. It binds the p40 protein subunit of IL-12 and IL-23, which are important in NK and T-cell activation.
One of the potential advantages of Vedolizumab is the decreased association of infections, especially serious infections. This may be owing to its mechanism, which has more targeted effect on the immune system than a TNF inhibitor. One caveat to interpreting the results was that prior exposure to a TNF inhibitor was allowed. This somewhats biases the results, as patients that have potentially failed a TNF inhibitor were included, though they were a minority. In subgroup analysis the results held when only evaluating patients that never had a TNF inhibitor. A downside vedolizumab of is its route of administration. Adalimumab can be given as weekly a subcuteanous injections at home.
For now there are multiple agents that can aid induce remission for moderate to severe UC. It is difficult to say that vedolizumab is first line for that purpose. Rather the decision is likely patient specific and there are multiple options that are superior to placebo (i.e. vedolizumab, adalimumab, and ustekinumab). It should be noted that corticosteroids are still the go to for disease activity severe enough to lead to hospitalization.
| F: Follow up | 52 weeks |
| R: Randomization | Yes |
| I: Intention to treat | Yes |
| S: Similar at baseline | Yes |
| B: Blinding | Yes |
| E: Equal treatment | Yes |
| S: Source (funding) | Takeda Pharmaceuticals |
- Harbord M, Eliakim R, Bettenworth D, et al.; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis. 2017 Jul 1;11(7):769-784.
- Irving P, Sands B, Hoops T, et. al. (2021). Ustekinumab versus adalimumab for induction and maintenance therapy in Moderate-to-Severe Crohn’s Disease: The SEAVUE study. Journal of Crohn’s and Colitis 2021; 15: S001-S002
- Khanna R, Zou G, Feagan BG. (2018). Evolution of the randomized controlled trial in inflammatory bowel disease: current challenges and future solutions. Inflammatory Bowel Disease 2018; 24(10):2155-2164
- Meier J, Sturm A. (2011). Current treatment of ulcerative colitis. World Journal of Gastoenterology 2011; 17(27):3204-3212.
