RAVE
“Rituximab versus Cyclophosphamide for ANCA-Associated Vasculitis”
by the Rituximab in ANCA-Associated Vasculitis-Immune Tolerance Network (RAVE-ITN) Research Group
N Engl J Med. 2010 Jul 15;363(3):221-32. [full text]
Granulomatosis with Polyangiitis (GPA) and Microscopic Polyangiitis (MPA) are ANCA-associated vasculitides most often affecting the kidneys and respiratory tract. They are historically associated with high rates of morbidity and mortality. In the 1960s, the combination of cyclophosphamide and steroids was introduced as a treatment and markedly improved outcomes (including mortality) in ANCA-associated vasculitis (e.g. GPA mean survival improved from about 5 months to several years, approaching survival rates of the general population). However, many patients still failed to achieve disease remission, often requiring prolonged courses of steroids, themselves not without complications. Additionally, cyclophosphamide is associated with many adverse effects such as infections, bladder injury, cancer, infertility, and cytopenias. Leading up to this 2010 trial, bench research had demonstrated the important role of B cells in the development of ANCA-associated vasculitis, and both anecdotal evidence and some small studies had suggested that rituximab – an anti-CD20 monoclonal antibody with anti-B cell effects – may be effective for ANCA-associated vasculitis.
Research Question
Is rituximab as effective (non-inferior to) as cyclophosphamide for induction of remission of severe ANCA-associated vasculitis?
Patient Population
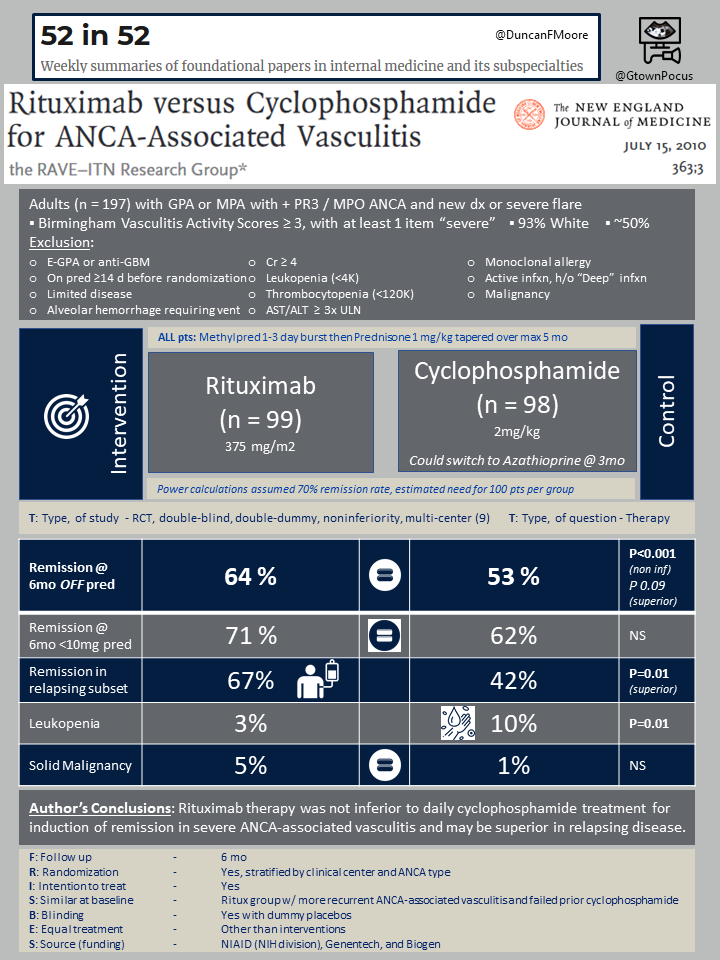
In this multicenter randomized double-blind double-dummy noninferiority trial 197 patients were recruited from nine medical centers from 2004-2008. All had severe ANCA-associated vasculitis, defined as biopsy proven GPA or MPA with a positive serum PR3-ANCA or MPO-ANCA and a Birmingham vasculitis activity score of at least 3. They were all patients who were otherwise being considered for treatment with cyclophosphamide and steroids.
The study had many exclusion criteria – among the most important were: eGPA or anti-GBM (Goodpasture), mild/moderate disease that would otherwise not warrant consideration of cyclophosphamide, history of severe diffuse alveolar hemorrhage requiring intubation, current infection, leukopenia (< 4K) or thrombocytopenia (< 120K), Cr > 4 (at the time of enrollment), underlying liver disease, HBV/HCV/HIV, recent treatment with steroids/rituximab/cyclophosphamide/PLEX, or known allergy to any monoclonal antibody. Baseline characteristics of the patients enrolled were: mean age 54, 46% female, almost entirely self-identifying as white (92%), 75% GPA and 24% MPA (newly diagnosed in 48%).
Intervention
Patients underwent 1:1 randomization to cyclophosphamide 2mg/kg + placebo (n=98) or rituximab 375 mg/m2 + placebo (n=99); both groups received methylprednisolone 1g for 1-3 days then a prednisone taper based on ongoing clinical and laboratory assessment of disease activity. The cyclophosphamide group could switch to azathioprine if they achieved disease remission after 3 months. All patients were followed for 6 months.
Outcomes and Results
The primary outcome was remission of disease off of prednisone at 6 months after enrollment (i.e. the outcome was only achieved if the patient did not require any steroids for disease control at 6 months). Secondary outcomes included remission of disease on prednisone < 10mg daily, rate of limited or severe disease flares per month, quality of life, ANCA negativity at 6 months, and treatment discontinuation (for any reason).
Baseline disease activity was similar between the two groups. The primary outcome of disease remission off of prednisone at 6 months was achieved by 64% of the rituximab vs 53% of the cyclophosphamide group (P < 0.001 for non-inferiority, non-significant for superiority). Non-inferiority with a trend toward superiority of rituximab held for both the GPA and MPA subgroups.
Among patients with a history of relapsing disease, rituximab achieved a higher rate of remission than cyclophosphamide (67% vs 42%, P = 0.01). No differences were found in rates of disease flare per month or quality of life measures. Interestingly, more patients in the rituximab group (47%) were found to be ANCA negative at 6 months compared to the cyclophosphamide group (24%; P = 0.004); this appeared to be driven entirely by PR3-ANCA negativity (as opposed to MPO-ANCA negativity). The primary outcome, however, did not differ by ANCA type. There was no significant difference in rates of overall total adverse events.
Discussion
Overall, rituximab was non-inferior to cyclophosphamide in achieving disease remission off of prednisone at 6 months, without any significant differences seen in multiple secondary outcomes including overall adverse events. These results were reproduced in the RITUXVAS trial (same treatment comparison but in patients with renal vasculitis). Notably, the authors point out that they had anticipated rituximab would lead to FEWER drug-associated adverse events than cyclophosphamide, i.e. it would be better tolerated. The ability to use a presumably safer medication (rituximab) to the same effect as cyclophosphamide was a part of the motivation for the study. The expected difference in incidence of side effects between the two medications was not seen, possibly due to limited (6 month) follow up period.
RAVE was criticized for its limited scope both in its inclusion/exclusion criteria (particularly exclusion of patients without severe disease, those with severe alveolar hemorrhage, and those with Cr > 4) and its short 6 month follow up period; these were seen as limiting the generalizability of the study as well as its ability to inform long-term treatment approach. Additionally, at baseline those in the rituximab group were more likely to have a history of recurrent ANCA-associated vasculitis and to have failed a prior trial of cyclophosphamide, introducing a potentially confounding selection bias. Despite its limitations, this trial was widely considered to provide compelling evidence for the efficacy of rituximab in severe ANCA-associated vasculitis. It led to FDA approval of rituximab plus steroids for the treatment of GPA and MPA.
Post by Jon Thaler
