IMPACT
Once-Daily Single-Inhaler Triple versus Dual Therapy in Patients with COPD.
Lipson DA, Barnhart F, Brealey N, et al; IMPACT Investigators.
N Engl J Med. 2018 May 3;378(18):1671-1680. [Full text]
Summary by Natalie Burkhart
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines are the major guidelines for Chronic Obstructive Lung Disease (COPD) management. They group disease severity categories, the “ABCD” groups (GOLD D is the most severe), and offer a stepwise framework for COPD therapy [1]. Inhaled controller options include anti-muscarinic antagonists (LAMA), combined long-acting β2-agnoist (LABA)-LAMA, or combined inhaled corticosteroids (ICS)-LABA.
For treatment refractory disease within Group D, those who have significant symptoms despite treatment with either ICS-LABA or LAMA-LABA, GOLD guidelines suggest triple therapy (ICS-LABA-LAMA) [2]. This recommendations first iteration in 2017 were based on limited large-scale randomized data to support the use of triple therapy compared to dual therapy [2], and that triple therapy was via separate inhalers, raising adherence concerns [3].
The Informing the Pathway of COPD Treatment (IMPACT) trial was the first large-scale randomized trial that compared the rates of moderate to severe COPD exacerbations in patients with symptomatic disease between once-daily single inhaled triple therapy (ICS-LABA-LAMA) and once-daily dual therapy (either ICS-LABA or LABA-LAMA).
Study Design and Patient Population
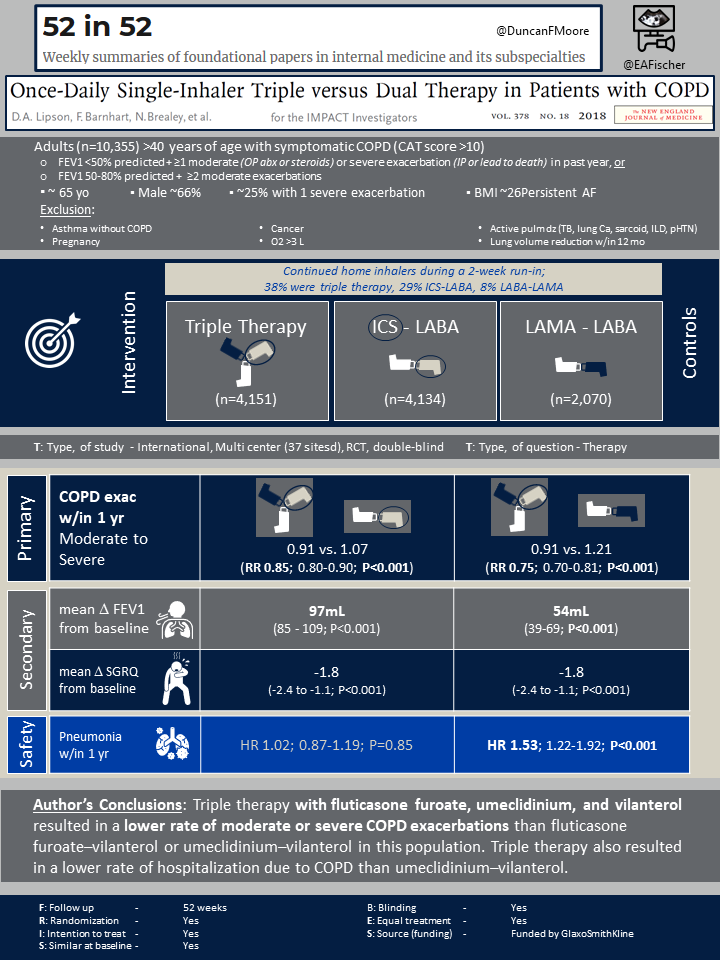
The IMPACT trial was a randomized, double-blind, phase III, multicenter trial performed in 37 countries that evaluated the rate of moderate or severe COPD exacerbations in patients receiving once-daily triple therapy with fluticasone furoate (ICS), umeclidinium (LAMA), and vilanterol (LABA) compared to once-daily dual therapy with fluticasone furoate-vilanterol or umeclidinium-vilanterol.
Patients aged 40 years or older with symptomatic COPD (determined by COPD Assessment Test (CAT) score >10) were eligible for the study. Additionally, patients had to have either (1) FEV1 <50% predicted and history of at least one moderate or severe COPD exacerbation in the prior year, or (2) FEV1 50-80% predicted with a history of either two moderate exacerbations or one severe exacerbation in the past year. Patients with asthma were included if they had a concurrent diagnosis of COPD.
Exclusion criteria included diagnosis of asthma without COPD, pregnancy, history of active pulmonary diseases (active TB, lung cancer, sarcoid, ILD, pulmonary hypertension), cancer, oxygen therapy >3L or lung reduction surgery in the past 12 months.
Patients were randomized in a 1:1:1 fashion to either ICS-LABA-LAMA (triple therapy), ICS-LABA, or LABA-LAMA. Prior to randomization, patients continued their home inhalers during a 2-week run-in period. Home inhalers included LABA, LAMA, ICS, and combination inhalers. Once randomized, the patients continued their treatment arm for a total of 52 weeks. All treatment arms were given a once-daily Ellipta inhaler. The trial was sponsored by the pharmaceutical company, GlaxoSmithKline.
Outcomes
The primary outcome was the annual rate of moderate or severe COPD exacerbations. Moderate exacerbations were defined as requiring outpatient antibiotics or corticosteroids. Severe exacerbations were defined as requiring hospitalization or resulting in death. Two co-primary treatment comparisons were made with triple therapy vs. ICS-LABA and triple therapy vs. LABA-LAMA.
Secondary outcomes included change from baseline FEV1 at week 52 and change from baseline St. George’s Respiratory Questionnaire (SGRQ) scores at week 52, which assessed health-related quality of life. Major safety outcomes included pneumonia, lower respiratory infections (excluding pneumonia), cardiovascular events, and urinary retention. Pneumonia was diagnosed based on infiltrate on chest x-ray.
Results
A total of 10,355 patients were randomized to either triple therapy (n=4,151), ICS-LABA (n=4,134), or LAMA-LABA (n=2,070) with intention-to-treat. There were no clinically significant differences in the baseline characteristics between the three study arms. Overall, the mean age was 65.3 with a male predominance of 66%. In the prior year, 47% of patients reported >2 moderate COPD exacerbations. Prior to staring the trial, 38% of patients were already receiving triple therapy (ICS-LABA-LAMA), 29% were receiving ICS-LABA, and 8% were receiving LABA-LAMA.
The annual rate of moderate or severe COPD exacerbations in triple therapy group was 0.91 per year compared to 1.07 per year the ICS-LABA group (rate ratio with triple therapy, 0.85; 95% CI 0.80-0.90; 15% difference; P<0.001). The annual rate of moderate or severe COPD exacerbations in LABA-LAMA group was 1.21 per year (rate ratio with triple therapy, 0.75; 95% CI 0.70-0.81; 25% difference; P<0.001).
When comparing the mean change from baseline FEV1, the difference between ICS-LABA-LAMA group and ICS-LABA group was 97mL (95% CI, 85 to 109; P<0.001), and the difference between ICS-LABA-LAMA group and LABA-LAMA group was 54mL (95% CI, 39 to 69; P<0.001). For mean change in baseline SGRQ score, the difference between ICS-LABA-LAMA vs. ICS-LABA was -1.8 (95% CI-2.4 to -1.1; P<0.001) and ICS-LABA-LAMA vs. LABA-LAMA was -1.8 (95% CI -2.6 to -1.0; P<0.001).
For adverse events, there was a higher incidence of pneumonia in the treatment groups that included an ICS vs. the LABA-LAMA group, with 95.8, 96.6, and 61.2 events per 1000 for triple therapy, ICS-LABA, and LABA-LAMA, respectively. The risk of pneumonia was significantly higher in ICS-LABA-LAMA vs. LABA-LAMA group (hazard ratio, 1.53; 95% CI 1.22 to 1.92; P<0.001). There was no statistically significant difference in risk of pneumonia between ICS-LABA-LAMA and ICS-LABA (hazard ratio, 1.02; 95% CI 0.87-1.19; P=0.85). There were no other statistically significant differences in adverse events among the groups.
Discussion
The IMPACT trial was the first large-scale, multicenter trial that demonstrated once-daily single-inhaled triple therapy (ICS-LABA-LAMA) resulted in statistically significant lower rates of moderate or severe COPD exacerbations in symptomatic patients compared to once-daily dual therapy with either ICS-LABA or LABA-LAMA. Additionally, patients receiving triple therapy had statistically significant improvement in FEV1 and improved quality of life after 52 weeks in comparison to patients receiving dual therapy.
This was also the first large-scale study that directly evaluated the different treatment options recommended by the GOLD 2017 guidelines for treatment refractory Group D. Prior to this study, multiple smaller studies had demonstrated improvement in airflow obstruction with addition of ICS-LABA to LAMA; however, there was limited data directly comparing ICS-LABA-LAMA, ICS-LABA, and LABA-LAMA [3].
Additionally, this was the first trial to administer all three treatment arms with a single inhaler. But the role of ICS in the treatment of COPD is somewhat conflicting. Similar a prior study [3], the IMPACT trial found a higher incidence of pneumonia in patients receiving ICS, with a statistically significant increase in cases of pneumonia in patients receiving triple therapy vs. LABA-LAMA. Yet the absolute risk of pneumonia is still low, with only 95.8 events per 1000 within the 1 year IMPACT study period. The FLAME trial showed benefit in LABA-LAMA over ICS-LABA [5]. Meanwhile, WISDOM, looked at stepping down triple therapy after 1 year (by removing the ICS) in severe COPD and found it to be non-inferior to continuing triple therapy [4].
Although this study is considered a landmark study in directly comparing Group D COPD treatment options, there are significant limitations that require the results be interpreted with caution. Prior to initiation of the trial, 38% of patients were already on triple therapy. Those who were already on triple therapy and were then randomized to either dual therapy group may have had a stepdown in therapy, resulting a possible increase in exacerbations. Patients who had concurrent asthma could enroll in the trial; however, the study did not list the number of patients with concurrent asthma. If a significant number of patients in the study had asthma, this could explain why ICS combined with LABA-LAMA had a significant reduction in COPD exacerbations compared to LABA-LAMA alone.
Since the IMPACT trial was released in 2018, multiple trials have examined if peripheral blood eosinophil levels can help indicate which COPD patients are more likely to respond to the addition of ICS [5]. In fact, a post hoc analysis of the IMPACT trial was released in 2019, with a significant decrease in COPD exacerbations for triple therapy vs. LABA-LAMA in patients with elevated blood eosinophil count [7]. Based on recent research involving peripheral eosinophil count, the GOLD 2020 guidelines recommend considering ICS-LABA-LAMA for patients with recurrent exacerbations and an eosinophil count >100 [1].
It should be noted that triple therapy options available are relatively new and the price can be prohibitive, though the intervention may be cost effective overall if exacerbations are avoided. Furthermore, the most recent GOLD guidelines from 2020 reflect the findings of this study and suggest triple therapy as a treatment for refractory group D COPD [1].
| F: Follow up | 52 weeks |
| R: Randomization | Yes |
| I: Intention to treat | Yes |
| S: Similar at baseline | Yes |
| B: Blinding | Yes |
| E: Equal treatment | Not discussed / by design |
| S: Source (funding) | GlaxoSmithKline; IMPACT ClinicalTrials.gov number, NCT02164513. opens in new tab. |
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2020 Report.
- Volgelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med 2017;195:557-82.
- Jung KS, Park HY, Park SY, et al. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respi Med 2012;106:382-9.
- Magnussen H, et al. for the WISDOM Investigators. Withdrawal of Inhaled Glucocorticoids and Exacerbations of COPD. N Engl J Med. 2014 Oct 2;371(14):1285-94.
- Wedzicha JA, Banergi D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med 2016;374:22222-34
- Bafadhel M, Peterson S, De Blas MA, et al. Predictors of exacerbation risk and response to budesonide in patients with chronic obstructive pulmonary disease: a post-hoc analysis of three randomized trials. Lancet Respir Med 2018;6(2):117-26
- Pascoe S, Barnes N, Bursselle G, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. The Lancet Respiratory medicine 2019;7(9): 745-56
