CheckMate-003 – Nivolumab Phase 1 Safety
Safety, activity, and immune correlates of anti-PD-1 antibody in cancer.
Topalian SL, Hodi FS, Brahmer JR, et al.
N Engl J Med. 2012 Jun 28;366(26):2443-54. [Full text]
Summary by Tina Roy
The immune system, particularly cytotoxic T cells, plays a key role in suppressing malignant cells. However, cancer cells have developed mechanisms to evade the immune system. In the last decade, advances in tumor immunology have led to breakthroughs in cancer therapy such as immune checkpoint pathway inhibitors. In 2011, the FDA approved anti-CTLA antibody ipilimumab for use in treating melanoma. This set the stage for further research and development in the sphere of cancer immunotherapy [1].
One important immunotherapy target is programmed death 1 (PD-1). Interactions between PD-1 and programmed death ligand 1 (PDL1) in the tissue periphery leads to immunosuppression. Therapy targeting the PD-1 checkpoint inhibits the immunosuppressive effect, thus resulting in antitumor activity [2]. Nivolumab was the first monoclonal antibody targeting PD-1 and its study led to further developments in cancer therapy for solid tumors [3].
RECALL: “Phase 1 trials are primarily designed to accumulate short-term safety (toxicity) and pharmacological data. Although preliminary efficacy may be addressed (“proof of concept” efficacy), it is a secondary endpoint. The numbers of patients are small, the numbers of patients receiving efficacious doses are very small, and controls are absent. Evaluation of efficacy and of long-term toxicity requires longer, larger, and controlled studies.”
Muglia JJ, DiGiovanna JJ. Phase 1 clinical trials. J Cutan Med Surg. 1998 Apr;2(4):236-41.
This study, CheckMate-003, was a phase 1 dose escalation study. that aimed to evaluate the safety and efficacy of nivolumab in diverse advanced solid tumors. It was a follow-up to an smaller pilot study of 39 patients assessing the safety and efficacy of nivolumab. Given favorable outcomes in the pilot, this multiple-dose, dose-escalation trial involving a larger patient population was established.
Patients
A total of 296 patients with advanced solid tumors, specifically melanoma, non-small-cell lung cancer (NSCLC), renal-cell cancer, castration-resistant prostate cancer, and colorectal cancer received anti-PD-1 antibody therapy.
Patients with advanced solid tumors, over the age of 18, greater than 12 week life expectancy, Eastern Cooperative Oncology Group (ECOG) performance status of 0-2 were included in the study if they had adequate hematologic, hepatic, and renal function and history of 1 to 5 prior systemic therapies.
Patients were excluded if they had history of chronic autoimmune disease, prior T-cell modulatory antibody therapy, immunosuppressive medications, or chronic infections including HIV, Hepatitis B, or Hepatitis C.
Efficacy and treatment populations had similar baseline characteristics. The average age was 63 years old and 66% of the patients were male. Of the patients in the study, almost half (47%) received at least three prior regimens. Notable prior regimens varied depending on tumor type and are listed below:
- Melanoma
- Immunotherapy (64%)
- BRAF inhibitors (8%)
- Non-small cell lung cancer
- Platinum based chemotherapy (94%)
- Tyrosine kinase inhibitors (34%)
- Renal cell cancer
- Nephrectomy (94%)
- Immunotherapy (59%)
- Antiangiogenic therapy (74%)
Intervention and Outcomes
The patients were administered nivolumab every 2 weeks for a 8-week treatment cycle. The patients were randomized into nivolumab cohorts with dosages ranging from 0.1 to 10 mg/kg. They received treatment for 2 years. Treatment was stopped if they experienced unacceptable adverse effects, progressive disease, or withdrew consent.
The primary endpoint was safety at varying doses and determination of the maximum tolerated dose. Secondary endpoints were to assess efficacy by CT imaging after each cycle (8 weeks). Progression free survival rate was assessed and tumor response was defined per RECIST criteria as indicated below for the target lesions:
- Complete response (CR): disappearance
- Partial response (PR): at least a 30% decrease in the sum of the largest diameter (LD)
- Stable disease (SD): neither a sufficient shrinkage or increase
- Progressive disease: at least a 20% increase in the sum of LD
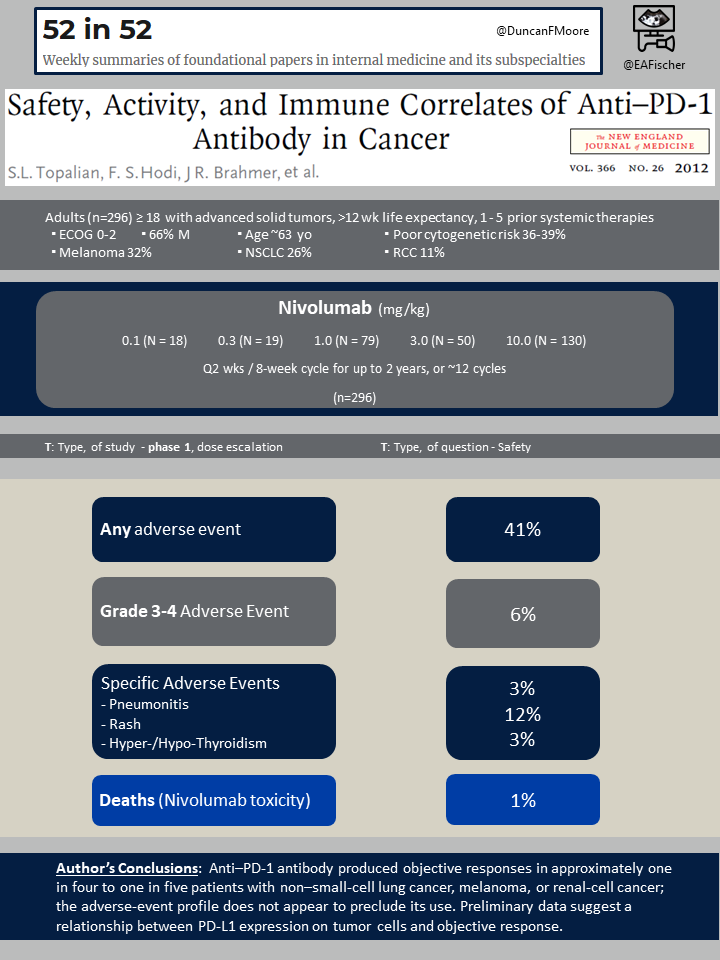
Results
A maximum tolerated dose was not reached based on the doses tested. Fifteen (5%) of the 296 patients discontinued treatment due to treatment related adverse events. Sixy-two patients (21%) died, mostly due to disease progression. The most common therapy related adverse events included fatigue, rash, diarrhea, pruritus, decreased appetite, and nausea. Grade 3 or 4 treatment-related adverse events were observed in 14% of the patients. The severity and frequency of adverse events were similar between doses.
Immune-related adverse events were observed including pneumonitis, vitiligo, colitis, hepatitis, hypophysitis, and thyroiditis. These adverse events were treated with immunomodulatory agents (steroids, infliximab, mycophenolate). There were three (1%) drug related deaths, all from immunotherapy related pneumonitis.
Objective responses (complete or partial response) were observed in patients with non-small cell lung cancer, melanoma, or renal cell cancer. For patients receiving any dose of nivolumab for melanoma, 28% (95% CI 19-38) had an objective response and 6% (95% CI 2-13) had stable disease and 41% PFS (95% CI 20-51) at 24 weeks. For patients receiving any dose of nivolumab for lung cancer, 18% (95% CI 11-29) had an objective response and 7% (95% CI 2-15) had stable disease and 21% PFS (95% CI 20-51) at 24 weeks. In lung cancer, the objective response was demonstrated in all non-small-cell histology types, but stable disease was only observed in non-squamous cell histology types. For patients receiving any dose of nivolumab for renal cell cancer, 27% (95% CI 13-46) had an objective response and 27% (95% CI 13-46) had stable disease and 56% PFS (95% CI 39-73) at 24 weeks.
No objective responses were observed in patients with colorectal or prostate cancer.
Discussion
This study was a phase 1, dose-escalation trial used to assess the safety and efficacy of PD1 inhibition. The study demonstrated that approximately 20-25% of patients had an objective response to nivolumab given any dose of therapy for non-small cell lung cancer, melanoma, or renal cell cancer. Given that this was a phase 1 trial, there was no comparison with a standard agent. However, per the authors, the initial data on progression free survival and response rates suggested improvements in durability compared to standard treatment.
At the time of this study (CheckMate-003) was published, immunotherapy was shown to have efficacy in melanoma and renal cell cancer. However, given the findings in non-small cell lung cancer this data instigated further exploration of immunotherapy use in other malignancies. The subsequent CheckMate series of studies explored the use of nivolumab to standard therapy in phase 3 trials. Other study series, such as KEYNOTE or IMpower studied the efficacy of other immune checkpoint inhibitors such as pembrolizumab and atezolizumab, respectively. These studies changed the landscape of treatment in oncology. For example, in NSCLC, PD1 axis inhibition has now become an integral part of therapy, not only for advanced tumors, but also as a first line agent for tumors that have >50% PDL1 expression [4].
The study also demonstrated therapy related toxicities unique to immunotherapy agents. These toxicities are now recognized as being autoimmune in nature and can affect essentially any organ. As demonstrated in CheckMate-003 and subsequent studies, these adverse effects can range from mild to severe and can lead to death. Given the potential severity, these adverse effects limit the use of immune-checkpoint inhibitors in some patients [5].
In conclusion, the CheckMate-003 trial was one of many trials that led to a new era in cancer therapy. Immunotherapy has led to improved outcomes in many solid tumors. However, continued efforts to better identify and treat immunotherapy related toxicities are necessary as immunotherapy becomes a backbone of guideline directed therapy.
| F: Follow up | 2 years (or until toxicity or progressive disease) |
| R: Randomization | Yes, dosing cohorts |
| I: Intention to treat | Yes |
| S: Similar at baseline | Yes |
| B: Blinding | NO, phase 1 trial |
| E: Equal treatment | Yes |
| S: Source (funding) | Bristol-Myers Squibb |
- Yang, Yiping. 2015. “Cancer immunotherapy: harnessing the immune system to battle cancer.” J Clin Invest 125, no. 9 (September): 3335-3337.
- Boussiotis, Vassiliki. n.d. “Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway.” N Engl J Med 375 (375): 1767-1778.
- Chen, Liepin, and Xue Han. 2015. “Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future.” J Clin Invest 125, no. 9 (September): 3384-3391.
- Doroshow, Deborah. 2019. “Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes.” Clin Cancer Res 25, no. 15 (March): 4592-4602.
- Johnson, Douglas. 2020. “Balancing Cancer Immunotherapy Efficacy and Toxicity.” J Allergy Clin Immunol Pract 8, no. 9 (Jun): 2898-2906.
