CLICK – Chlorthalidone in Advanced CKD
Chlorthalidone for Hypertension in Advanced Chronic Kidney Disease.
Agarwal R, Sinha AD, Cramer AE, Balmes-Fenwick M, Dickinson JH, Ouyang F, Tu W.
N Engl J Med. 2021 Nov 5. [Full text]
It is well known that controlling blood pressure (BP) has cardiovascular benefits. Seminal works in the field of hypertension (HTN), for example ALLHAT or SPRINT, utilized a thiazide, specifically chlorthalidone, to achieve such results. Owing to this and other work, thiazides are a first line for new HTN; yet, often they are not considered for HTN in severe disease or with advanced co-morbidities, like chronic kidney disease (CKD). In fact, KDOQI guidelines have recommended against thiazides in CKD4 or worse as “thiazide diuretics are of minimal effectiveness for [extracellular fluid volume] ECF reduction at low levels of GFR, [therefore] a loop diuretic is preferred for this purpose in patients with GFR less than 30 mL/min/1.73 m2.” [1] But does decreased ECF reduction equate to decreased efficacy controlling BP?
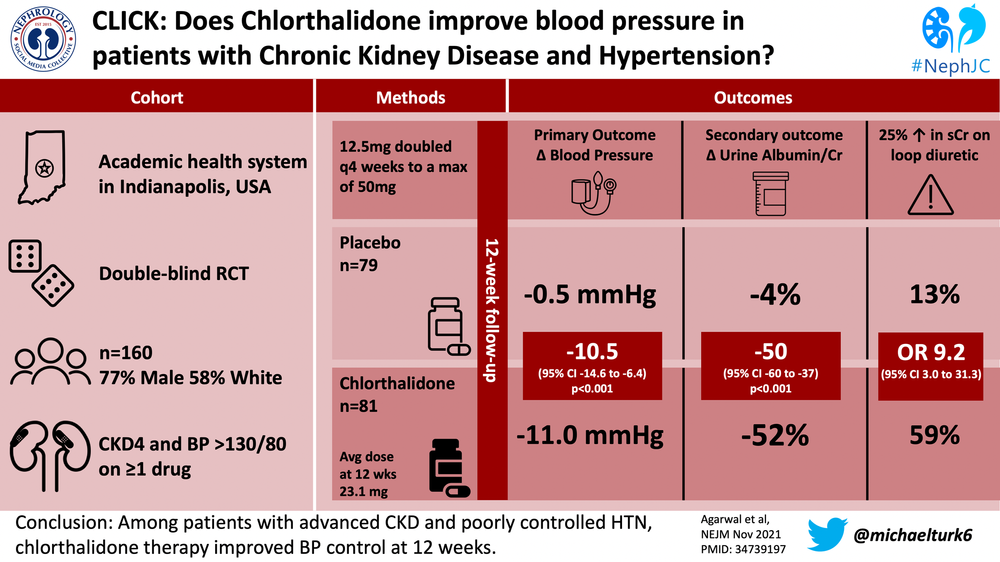
The Chlorthalidone in Chronic Kidney Disease (CLICK) Trial was a double blind, randomized, placebo-controlled trial that aimed to revist the use of chlorthalidone in CKD 4 and establish its viability as an anti-hypertensive.
Patient population
The target population had CKD 4 (GFR 15 to <30) and uncontrolled HTN and was recruited from 3 institutions in Indiana. Uncontrolled HTN was confirmed by 24-hour ambulatory BP monitoring after a medication standardization period and defined as a mean 24-hour ambulatory SBP ≥ 130 mm Hg or DBP ≥80 mm Hg while receiving antihypertensives.
Patients were excluded if the 24-hour ambulatory SBP was ≥160 mm Hg or DBP ≥100 mm Hg, had prior stroke or myocardial infarction, had been hospitalized for heart failure within 12 weeks before randomization, were receiving high-dose loop diuretics (>200 mg furosemide or >100 mg torsemide daily), or had received a thiazide or a thiazide-like diuretic within 12 weeks before randomization.
Design and Outcomes
Potentially eligible patients had a 2 week standardization period in which patients were transitioned to “preferred” BP anti-HTN agents. All patients were placed on at least an ACEI (Lisinopril) or ARB (Losartan) unless there was a contraindication. The preferred b-blocker was Atenolol, the preferred CCB was amlodipine, and the preferred diuretic was torsemide. Any additional agents (i.e. clonidine) continued. All patients were given written instructions to consume less than 100 mmol of sodium per day.
Patients were randomly assigned 1:1 to receive either chlorthalidone or placebo, stratified by previous use of loop diuretics. Those receiving chlorthalidone were started at 12.5 daily. If the home SBP was ≥ 135 mm Hg or DBP ≥ 85 mm Hg, the dose was doubled to 25 mg daily at the 4 week follow-up and then 50 mg daily at the 8 week follow-up, if necessary. The dose was not increased if the patient had symptomatic orthostatic hypotension, hypercalcemia, hypokalemia (K<3), acute gout, or poorly controlled diabetes.
The primary outcome was the change in 24-hour ambulatory SBP from baseline to 12 weeks. Secondary outcomes were the change from baseline to 12 weeks in the urinary albumin-to-creatinine ratio, NT-proBNP level, plasma renin and aldosterone levels, and total body volume.
Results
Only 1519 of 2849 screened were potentially eligible, with the most frequent eligibility exclusions being a BP out of range or recent thiazide use. Only 403 actually consented but many more subsequently withdrew or were excluded during the pre-trial period, leaving 160 for randomization. The average age was ~66, ~77% were male, ~58% white and ~40% black, and ~76% had diabetes. Patients were on an average of 3.4 anti_HTN medications, with 60% on concurrent loop diuretics.
The adjusted change in 24-hour ambulatory SBP from baseline to 12 weeks was −11.0 mm Hg in the chlorthalidone group and −0.5 mm Hg in the placebo group (mean difference −10.5 mm Hg; 95% CI, −14.6 to −6.4; P<0.001). The 24-hour ambulatory DBP from baseline to 12 weeks was −4.9 mm Hg and −1 mm Hg, chlorthalidone vs. placebo (mean difference −3.9 mm Hg; 95% CI, −6.3 to −1.5). The chlorthalidone group at 4, 8 and 12 weeks, compared to the placebo group had lower seated clinic SBP for a △SBP (between group) of −11.9 mm Hg, −15.7 mm Hg and −15.1 mm Hg, respectively.
The urinary albumin-to-creatinine ratio was lower in the chlorthalidone group throughout the study and 2 weeks after the regimen was discontinued was −38% in the chlorthalidone group compared to −6% in the placebo group (between-group difference, −34%; 95% CI, −48 to −16). A similar result was noted for the NT-proBNP, −14% in the chlorthalidone group and +8% in the placebo group 2 weeks after discontinuation,
Known metabolic derangements associated with thaizaide use (hypoK, hypomag, hypoNa, hyperglycemia, hyperuricemia, etc.) were more common with chlorthalidone, but serious events were rare and only 4 in the chlorthalidone group required discontinuation compared to 1 in the placebo group.
Discussion
While perhaps thiazides are less effective as diuretics in advancing CKD, CLICK dispels the dogma that chlorthalidone is less effective at controlling BP in this population. The SBP decrease of 11 mm Hg that can be attributed to this single agent is quite robust, especially considering patients were already on an average of 3.4 other anti-HTN agents. The significant finding of decreased urinary albumin-to-creatinine ratio with chlorthalidone also suggest additional cardiovascular benefits.
Is this a class effect or specific to chlorthalidone? Chlorthalidone’s reported half-life is 45 to 60 hours, while that of hydrochlorothiazide (HCTZ) is much shorter, 6 to 15 hours. This substantial difference does seem to favor chlorthalidone. At the least, extrapolating the results from chlorthalidone observed in CLICK to HCTZ should be done with caution.
This trial included 10 visits over 17 weeks, potentially closer follow-up than what is achieved in clinical practice. Thiazide side effects, especially metabolic ones, are common and were unsurprisingly observed here. Fortunately few were significant, though the frequency of follow-up may have mitigated the degree of any adverse effects. Therefore, while chlorthalidone can be used safely in CKD4, close monitoring is critical, especially while on con-current ACEI/ARB and/or loop diuretic. In summary, CLICK confirms both the efficacy of chlorthalidone for BP control in CKD4 and its safe administration, if cognizant of the common metabolic adverse effects.
| F: Follow up | 17 weeks: 4 visits in 3-weeks before randomization, 8 visits over 12-week study period, a final visit 2 weeks after assigned regimen discontinued |
| R: Randomization | Yes, stratified by concurrent diuretic use |
| I: Intention to treat | Yes |
| S: Similar at baseline | Yes |
| B: Blinding | Yes |
| E: Equal treatment | Yes |
| S: Source (funding) | National Heart, Lung, and Blood Institute and the Indiana Institute of Medical Research |
- Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004 May;43(5 Suppl 1):S1-290.