DONATE HCV: Transplants from HCV-Infected Donors
Heart and Lung Transplants from HCV-Infected Donors to Uninfected Recipients.
Woolley AE, Singh SK, Goldberg HJ, et al.; DONATE HCV Trial Team.
N Engl J Med. 2019 Apr 25;380(17):1606-1617. [Full text]
Summary by Palak Patel
As the need for heart and lung transplants increases, demand for organ availability increases as well. Within the United States, there have been significant limitations in regard to the availability of donor organs for both heart and lung transplants. One way to increase the donor pool would be to consider hepatitis C (HCV)-infected donors.
DONATE_HCV was published in 2019 and aimed to assess whether successfully treating recipients of HCV-infected donor organs was safe to potentially allow for a wider donor pool moving forward. Prior to the advent of a treatment for HCV, the majority of patients who received infected donor organs would develop chronic hepatitis C infection, with some developing significant liver disease with increased mortality. The creation of various direct-acting antiviral agents for hepatitis C may allow for the transplantation of HCV-infected donor organs.
The Donors of HCV nucleic acid amplification test (NAT) Positive Thoracic Allografts for Transplantation Evaluation in Non-HCV Recipients (DONATE HCV) trial studied the virologic response in those without HCV who received HCV-infected donor organs.
Sofosbuvir/velpatasvir (also Epclusa), is a known anti-viral therapy in the treatment of hepatitis C effective against all HCV-genotypes and has no major drug interactions with common post-transplant immunosuppressants. If transmission of HCV infection was prevented by administering a direct-acting antiviral therapy within hours after transplantation, hearts and lungs from HCV positive donors might be safely transplanted into uninfected recipients.
Patient population and Design
This was an open-label pilot trial at Brigham and Women’s Hospital in Boston. Patients were eligible if they were 18 and older and had an active waiting list status for heart or lung transplantation. They also must be willing to receive an organ from a donor with active HCV infection. Participants were ineligible if they were HCV antibody–positive, HBsAg-positive, had creatinine clearance <30 ml/min, had undergone prior transplantation, or was in inactive status on waiting list.
The patients would receive a 4-week regimen of sofosbuvir 400 mg once daily plus velpatasvir 100 mg once daily. THis was started immedietely after tranplantation.
From March 1, 2017, to July 31, 2018, a total of 217 patients were screened to participate in the trial and were assessed for eligibility. The trial was stopped early as the first 13 patients with 6 months post transplant follow up all had a sustained virologic response. By then a total of 44 patients had been enrolled, 36 of which had received lung transplants and 8 of which received heart transplants.
Outcomes
The primary outcome of the trial was weeks to sustained virologic response estimated after completion of four weeks of anti-viral therapy and graft survival estimated from time of transplantation.
Multiple secondary outcomes were observed including 1) hepatitis C viral load 2) death at 1 month, 6 months, and 12 months after transplantation, 3) hepatitis C antibody levels 4) the safety and side-effect profile of direct-acting antiviral regimens in the lung-transplant and heart-transplant recipients, 5) liver function tests, 6) the incidence of acute cellular rejection in the allograft for which treatment was indicated, and 7) mortality after transplantation were compared in all patients who received hearts and lungs during the same period from donors who did not have HCV infection.
In regards to safety monitoring, adverse events were described as grade 3, grade 4, and serious adverse events.
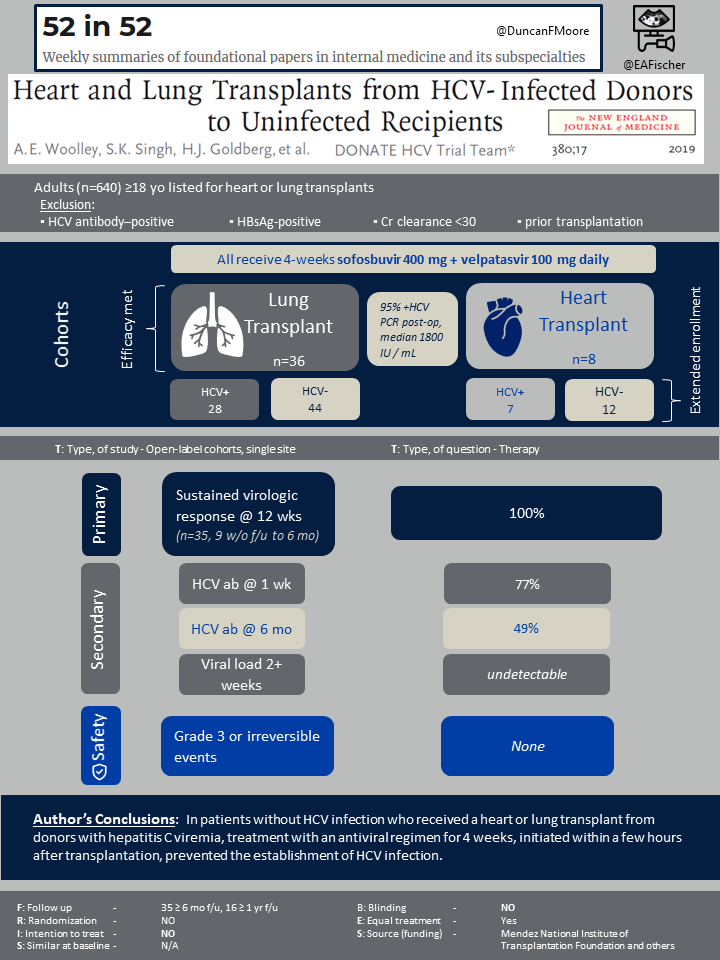
Results
Of the 44 initial recipients, 42 (95%) had a detectable hepatitis C viral load immediately after transplantation.
The primary outcome of the study demonstrated that four weeks of a direct-acting anti-viral therapy in HCV-infected donors initiated within a few hours of transplantation resulted in sustained virologic response 16 weeks after transplantation, which is 12 weeks after the four-week treatment course was completed. The trial also found that those who received HCV-infected donor organs had graft survival 6 months after transplantation.
Secondary outcomes showed that 27 of the 35 recipients (77%) had positive HCV-antibody tests within 1 week after transplantation, and 17 of the 35 recipients (49%) continued to have positive HCV-antibody tests 6 months after transplantation. All recipients had an undetectable hepatitis C viral load by approximately week 2 and remained undetectable. When comparing those who received a HCV-infected donor organ versus those who did not receive a HCV-infected donor organ, there was no overall significant difference in regard to outcomes; however, wide confidence intervals were observed. Low-grade acute cellular rejection that required treatment between HCV-positive donor organ recipients compared to recipients of a HCV-negative donor organ was not significantly different..
When assessing adverse events, it was determined by the investigators and the data and safety monitoring board that no adverse events were related to the trial medication and that no irreversible grade 3 or higher adverse events or serious adverse events were due to HCV infection. None of the HCV-infected organ recipients died in the first 6 months. One of the patients who received a heart transplant died at 8 months.
Discussion
Treating recipients of an HCV-infected donor with an agent that treats all HCV genotypes, even with a short course of 4 weeks, appears to prevent chronic HCV infection in those transplant patients. These results support the transplantation of HCV-infected donor heart and lungs to non-HCV infected patients. Additional studies have been conducted since 2019 that have demonstrated similar findings as discussed below. A 2020 study also found that there were no differences in outcomes of those who received HCV-infected donor organs versus non-infected donor organs [1].
Though the study demonstrated no major adverse effects (grade 3 or higher) the follow up time observed from time of transplant was short (i.e., 6 months to 1 year) and varied from patient to patient. More long-term data post transplantation is needed before large changes can be implemented. If further studies can replicate this safety data, treatment with a direct acting anti-viral therapy in HCV-infected donor recipients could allow for similar outcomes for both heart and lung transplant patients regardless of donor status.
This trial focused on the utilization of sofosbuvir/velpatasvir which can be used for any genotypes. Other HCV therapies should considered. As of 2020, 28% of centers are performing cardiac transplants using HCV+ donors. This is one of the studies that has changed the paradigm of transplantation and allow many of those awaiting an organ transplant have a higher chance of receiving an organ. Ongoing trials are being conducted and hopefully with further supportive findings, more transplant centers will begin utilizing HCV-infected donor organs in heart, lung, and other organ transplants until it is the standard of care.
| F: Follow up | 35 patients had at least 6 months of follow-up, and 16 patients had at least 1 year of follow-up. |
| R: Randomization | NO |
| I: Intention to treat | N/A |
| S: Similar at baseline | N/A |
| B: Blinding | NO |
| E: Equal treatment | Yes |
| S: Source (funding) | Mendez National Institute of Transplantation Foundation and others |
- Kilic A, Hickey G, Mathier M, Sultan I, Gleason TG, Horn E, Keebler ME. Outcomes of Adult Heart Transplantation Using Hepatitis C-Positive Donors. J Am Heart Assoc. 2020 Jan 21;9(2):e014495. doi: 10.1161/JAHA.119.014495. Epub 2020 Jan 8. PMID: 31910781; PMCID: PMC7033844.
