AUGUSTUS
Antithrombotic Therapy after Acute Coronary Syndrome or PCI in Atrial Fibrillation.
Lopes RD, Heizer G, Aronson R, et al; AUGUSTUS Investigators.
N Engl J Med. 2019 Apr 18;380(16):1509-1524. [Full text]
Summary by Tad Umali
Dual anti-platelet therapy (DAPT) has been the mainstay of therapy to prevent stent thrombosis and ischemia after percutaneous coronary intervention (PCI). Many patients that have required PCI also have atrial fibrillation and an indication for anti-coagulation, but “triple therapy” clearly carries increased bleeding risk. This study, AUGUSTUS, came on the heels of to two large studies (PIONEER AF-PCI and RE-DUAL PCI) that already examined novel oral anticoagulants (NOAC) in this context. So why was another study needed?
PIONEER AF-PCI compared three groups (rivaroxaban + P2Y12 versus lower dose rivaroxaban + P2Y12 + aspirin versus vitamin K antagonist + dual anti-platelet) and showed that the rivaroxaban groups had significantly lower incidence of bleeding with no significant increase in incidence of stroke, ischemic event, or stent thrombosis. RE-DUAL PCI examined three groups as well (dabigatran + P2Y12 versus lower dose dabigatran + P2Y12 versus vitamin K antagonist + dual anti-platelet) and showed similar results favoring dabigatran over vitamin K antagonist.
Based on these results, it was not clear whether the lower bleeding risk was owing to the standard or reduced doses of NOACS or to the removal of aspirin therapy. AUGUSTUS assessed the safety and efficacy of standard-dose apixaban compared with a vitamin K antagonist (VKA) and of low-dose aspirin as compared with placebo over 6 months in patients with atrial fibrillation and recent acute coronary syndrome (ACS) or PCI.
Patient Population and Design
Inclusion criteria included: age >18 years; atrial fibrillation with planned long-term oral anticoagulation; recent ACS or PCI, if an index event; planned use of P2Y12 inhibitor for at least 6 months. Key exclusion criteria involved primarily if the anticoagulation was for another condition such as a prosthetic valve, venous thromboembolism, or mitral stenosis but also severe renal insufficiency.
The design was a randomized in a 2×2 factorial design. Patients underwent randomization within 14 days after having an ACS or undergoing PCI. Randomization was stratified according to indication, ACS vs. PCI. There were four groups: (1) apixaban with aspirin, (2) apixaban with placebo, (3) warfarin with aspirin, and (4) warfarin with placebo. The apixaban with vitamin K antagonist comparison was open-label; however, the regimen comparing aspirin with matching placebo was double-blind. at enrollment.
Apixaban dosing was standard 5 mg twice daily (2.5 mg if 2 of age, weight or criteria were met). VKA target was the typical 2.0 to 3.0 and the aspirin dose was 81 mg.
Outcomes
The primary outcome, a safety outcome, was the composite of major bleeding (bleeds resulting in death, occurring in a critical organ, requiring 2 units of packed RBCs, or a hemoglobin drop of 2g/dL) or clinically relevant non-major bleeding (bleeds requiring medical attention or a change in anti-thrombotic therapy).
The secondary efficacy outcomes included the composite of death or hospitalization and the composite of death or ischemic events (stroke, myocardial infarction, stent thrombosis [definite or probable], or urgent revascularization). These comparisons were made between apixaban to a VKA as well as separately between aspirin and placebo.
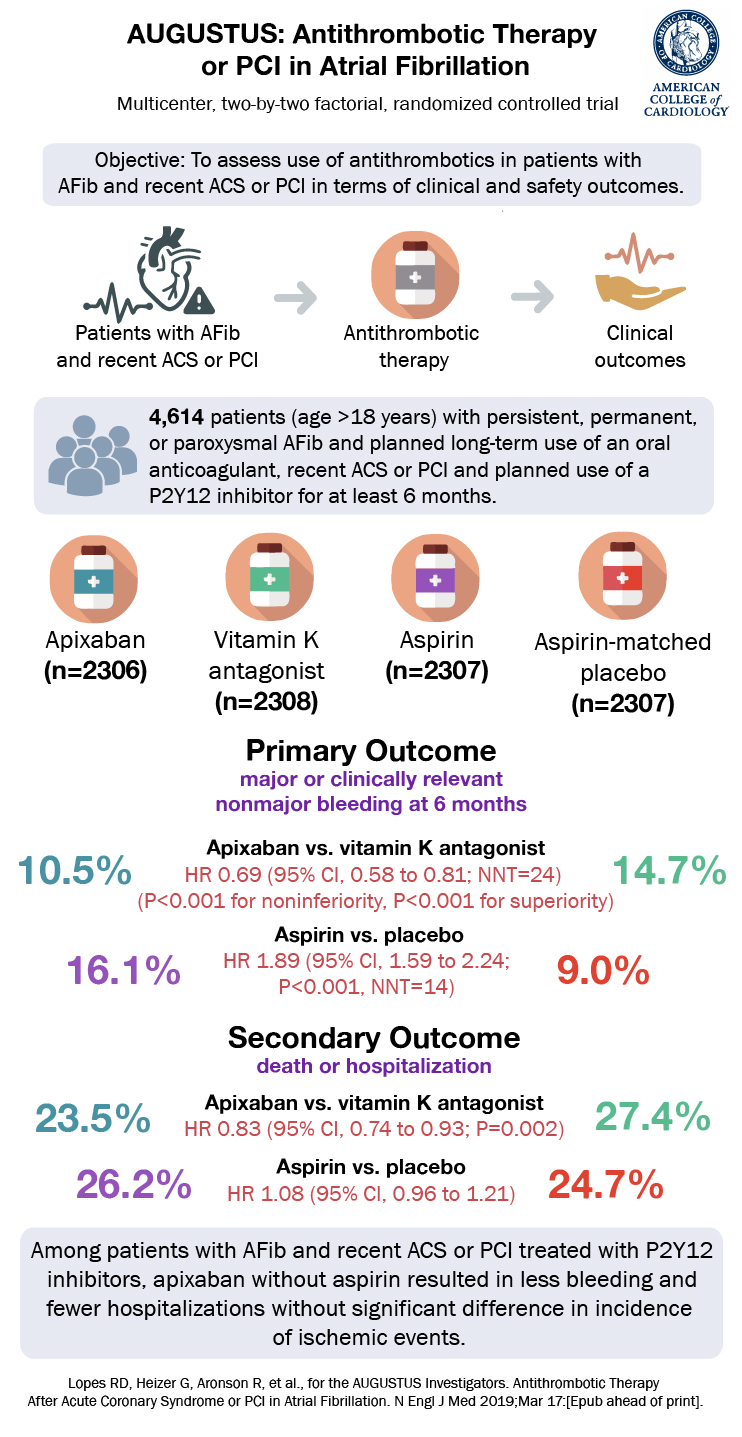
Results
A total of 4614 patients from 33 countries were enrolled. The median age was 70 years; 29% were female; 8.2% were Black/African American or belonged to another ethnic group; 49% of all patients had prior use of an oral anticoagulation agent; and median CHADS-VASc score was 3.9. Qualifying index events were equal across all arms as well – 37.3% of patients had ACS managed with PCI, 23.9% had ACS event managed medically, and 38.8 of patients had an elective PCI.
Apixiban had less major or clinically nonmajor bleeding events compared to a VKA: 10.5% vs. 14.7% (HR 0.69; 95% CI 0.58-0.81; P<0.001). Meanwhile, aspirin had more major or clinically nonmajor bleeding events compared to placebo: 6.1% vs. 9.0% (HR 1.89; 95% CI, 1.59 to 2.24; P<0.001).
For the secondary outcomes, apixiban had less deaths or hospitalizations compared to a VKA: 23.5% vs. 27.4% (HR 0.83; 95% CI 0.74-0.93; P=0.002) but there was no difference in the composite of deaths or ischemic events. Between aspirin and placebo there was no difference in either composite secondary outcome.
When grouped, a primary bleeding outcome event was highest among those receiving a VKA and aspirin (18.7%) and lowest among those receiving apixaban and placebo (7.3%). There were numerous prespecified subgroups with similar trends to the overall results. Of note, the time in the therapeutic range for VKAs was 59%, low but consistent with prior studies and real world practice.

Discussion
The AUGUSTUS trial with its 2×2 factorial design, showed that apixaban had less bleeding than a VKA, each combined with a P1Y12 inhibitor, for patients after ACS or PCI with atrial fibrillation. It also showed that aspirin led to more bleeding than placebo in the same population. In regards to the secondary efficacy outcomes apixiban was superior or at least non-inferior. This suggests that a regimen of a P2Y12 inhibitor plus apixaban, without aspirin was safe from bleeding perspective and appeared clinically effective. But AUGUSTUS was not designed or powered to compare a P2Y12 inhibitor + apixaban + placebo to P2Y12 inhibitor + apixaban + aspirin directly.
The authors noted a greater number of coronary ischemic events among those that did not take aspirin, even if the events were low and non significant. Patients could be enrolled up to 14 days after the ACS or PCI event, which is notable as the highest risk period for coronary ischemic events is in the first few days or weeks. Likely the takeaway is that a modest increase in coronary ischemic events may be the trade off for withholding aspirin, which clearly had a significant increased bleeding risk.
On a population basis those that have coronary artery disease and atrial fibrillation is already sizable and as the overall longevity in the developing world increases, that cohort is likely to increase. For these patients it is critical to know how to balance their bleeding risk with prevention of future events and AUGUSTUS adds to evidence to aid that decision making.
| F: Follow up | 6 mo + an additional visit at 7 mo |
| R: Randomization | Yes |
| I: Intention to treat | NO |
| S: Similar at baseline | Yes |
| B: Blinding | NO*, Open label for VKA vs apixaban; double blinding for ASA vs placebo. |
| E: Equal treatment | Yes |
| S: Source (funding) | Bristol-Myers Squibb and Pfizer |
