SCALE – Liraglutide for Weight Management
A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management.
Pi-Sunyer X, Astrup A, Fujioka K, et al.; SCALE Obesity and Prediabetes NN8022-1839 Study Group.
N Engl J Med. 2015 Jul 2;373(1):11-22. [Full text]
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) increase glucose-dependent insulin secretion and decrease inappropriate glucagon secretion, delay gastric emptying and increase satiety. Liraglutide, a GLP1 RA has been evaluated in multiple trials with benefits for diabetics in glycemic control and weight management [1]. A smaller precursor to the current study noted a dose dependent weight benefit to obese patients without diabetes [2]. This was an attempt to validate those findings in a larger population, specifically looking at 3.0 mg of liraglutide, injected once daily, as an adjunct to a reduced-calorie diet and increased physical activity for weight
management in overweight or obese adults without diabetes.
Patients and Intervention
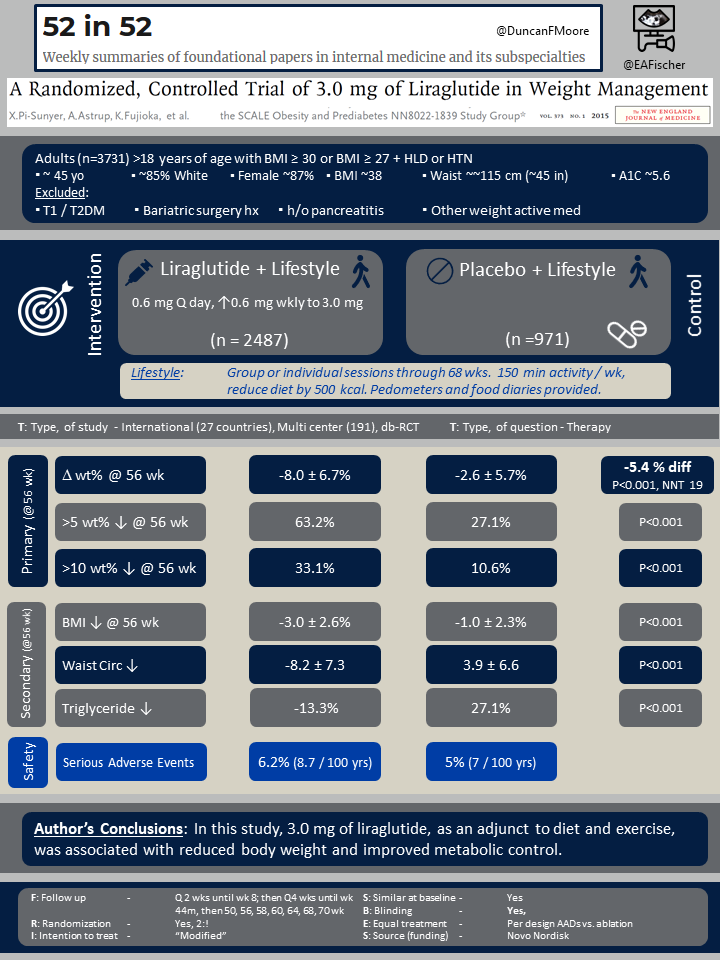
This was an international, multi center randomized trial representing 27 countries and 191 centers. Adults >18 years of age were were eligible if they either had a BMI ≥ 30 or BMI ≥ 27 and had dyslipidemia or hypertension.
Patients were excluded if they had either type 1 or type 2 diabetes, had prior bariatric surgery, had a history of pancreatitis or were on any other medication with a significant effect on weight (i.e. corticosteroids, certain anti depressants like TCAs, atypical antipsychotics and metformin, topiramate, phenteremine, etc.). Patients could not have an uncontrolled thyroid disorder, recent major depressive disorder or recent suicidal ideation or or prior suicide attempt.
Patients were randomized 2:1 to either liraglutide and lifestyle intervention or placebo with the same lifestyle intervention. Liraglutide was dosed starting at 0.6 mg weekly and increased dby 0.6 mg weekly to a goal of 3.0 mg. The lifestyle intervention consisted of group or individual sessions that continued through 68 weeks. Patients were recommended to do 150 minutes of activity weekly and to reduce their diet by 500 kcal. Pedometers and food diaries provided to all.
Outcomes
There were three prespecified co-primary end points: (1) weight change from baseline, (2) weight loss of at least 5% of baseline, and (3) weight loss of at least 10% of baseline body weight, all assessed at the 56 week mark
Major secondary end points included changes from baseline in BMI, waist circumference, glycemic control variables, cardiometabolic biomarkers, and health-related quality of life. Serious adverse events were tracked, including special attention to potentially drug specific effects such as pancreatitis.
Results
A total of 3,731 patients were enrolled, 2,487 in the liraglutide group and 971 in the placebo group. The groups were similar at baseline and had a mean age of ~45 years, were ~85% white, ~87% female, had an average BMI ~38, waist circumference of ~45 inches and an A1C of 5.6.
All co-primary outcomes favored the liraglutide group at 56 weeks, including the change in body weight (%): -8.0 versus -2.6 (△-5.4, 95% CI -5.8 to -5.0; P<0.001; NNT=19), weight loss > 5%: 63.2% versus 27.1% (P<0.001; NNT=3) or weight loss > 10%: 33.1% versus 10.6% (P<0.001; NNT=5).
Multiple secondary outcomes favored the liraglutide at 56 weeks as well. For example, there was ↓ BMI of -3.0 versus -1.0 (△ -2, 95% CI -2.2 to -1.9; P<0.001) and ↓ waist circumference (cm) -8.2 versus -3.9 (-4.2 95% CI -4.7 to -3.7; P<0.001). Additionally A1C and lipid profiles (especially triglycerides) were more improved with liraglutide.
Discussion
GLP-1 RAs do not cause hypoglycemia and improve glycemic control and weight management in diabetics. Furthermore, they have been shown to reduce nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes in trials that mostly included participants with type 2 diabetes and established cardiovascular disease [3]. In this large 2015 study, Pi-Sunyer et al. showed that similar weight benefits are seen in non-diabetics as well, a direct cardiovascular risk factor, so we hope similar cardiovascular benefits would be seen in non diabetics.
There are multiple agents associated with weight loss, often their effects are not durable, with weight gain after months after an intervention. A recent study has shown that liraglutide can maintain weight loss for an additional year after the initial drop [4]. Liraglutide or other GLP-1 RAs should be strongly considered for overweight and obese patients that have failed lifestyle interventions alone. Unfortunately for now, cost may be the major barrier to more widespread adoption.
| F: Follow up | Q 2 wks until wk 8; then Q4 wks until wk 44m then wks 50, 56, 58, 60, 64, 68, &70 |
| R: Randomization | Yes, 2:1, stratified by prediabetes status and BMI (≥30 vs. <30) |
| I: Intention to treat | Not explicitly stated |
| S: Similar at baseline | Yes |
| B: Blinding | Yes |
| E: Equal treatment | Not discussed except lifestyle intervention in both groups |
| S: Source (funding) | Novo Nordisk |
- Rigato M, Fadini GP. Comparative effectiveness of liraglutide in the treatment of type 2 diabetes. Diabetes Metab Syndr Obes. 2014 Mar 18;7:107-20.
- Astrup A, Carraro R, Finer N, et al.; NN8022-1807 Investigators. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond). 2012 Jun;36(6):843-54.
- Kalyani RR. Glucose-Lowering Drugs to Reduce Cardiovascular Risk in Type 2 Diabetes. N Engl J Med. 2021 Apr 1;384(13):1248-1260.
- Lundgren JR, Janus C, Jensen SBK, Juhl CR, Olsen LM, Christensen RM, Svane MS, Bandholm T, Bojsen-Møller KN, Blond MB, Jensen JB, Stallknecht BM, Holst JJ, Madsbad S, Torekov SS. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N Engl J Med. 2021 May 6;384(18):1719-1730.
