focuSSced – Tocilizumab for Sclerosis Associated ILD
Tocilizumab Prevents Progression of Early Systemic Sclerosis Associated Interstitial Lung Disease
Roofeh D, Lin CJF, Goldin J, et al. on behalf of the focuSSced investigators
Arthritis Rheumatol. 2021 Feb 3. [Full Text]
Summary by Saloni Godbole
Interstitial lung disease (ILD) is one of the most common causes of death in patients with systemic sclerosis. Until recently, treatment involved immunosuppression, such as with mycophenolate and cyclophosphamide and was limited to patients with clinically advancing disease. In 2020, Nintedanib (an antifibrotic medication) was approved for use in scleroderma associated ILD. More recently in March 2021, the FDA approved Tocilizumab (anti-IL-6) for systemic sclerosis-associated ILD, making it the first biologic drug approved for this indication. The approval was based on the focuSSced trial, which was originally designed to investigate Tocilizumab’s effect on scleroderma skin manifestations [1]. This study is a post-hoc analysis of the focuSSced trial as it pertains to lung disease and here we comment on its implications for clinical practice.
Patient Population
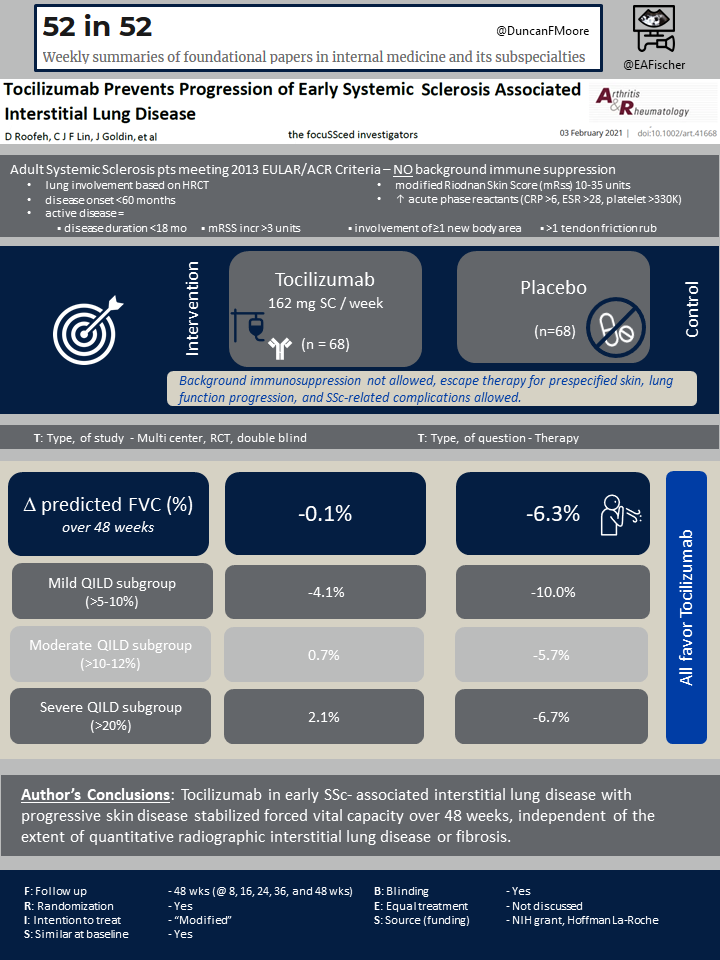
The original focuSSced study enrolled 210 participants: 106 were randomized into the placebo arm and 104 into the tocilizumab arm. All participants met the 2013 ACR/European League classification criteria for systemic sclerosis. They had modified Rodnan Skin Scores (mRss) between 10 and 35 units and early progressive skin disease with diffuse cutaneous involvement. Participants also had elevated acute phase reactants (CRP >6mg/L, ESR >28mm/h, or platelet count >330×10^9/L) and active disease as defined by disease duration less than or equal to 18 months or recent mRSS increase. Background immunosuppressive therapy was not allowed [1].
In this post-hoc analysis of a phase III trial for progressive skin disease, the presence of lung disease was not required for enrollment. Importantly, patients without lung involvement were subsequently excluded from the post-hoc analysis. Out of the 210 initial participants, 65% (136) had ILD based on high-resolution CT scan (HRCT), 68 received Tocilizumab and 68 placebo.. These participants were further stratified into four categories based on amount of lung involvement and fibrosis on HRCT scan: minimal (<5%), mild (>5-10%), moderate (>10-20%), and severe (>20%). The majority of participants (77%) had moderate to severe involvement. There were no baseline significant differences between the tocilizumab and placebo arms.
Outcomes
The primary outcome measure for the focuSSced trial was change in Modified Rodnan Skin Score (mRSS) from baseline to week 48. The major secondary outcome was change from baseline in percent predicted forced vital capacity (FVC). The most common adverse events in tocilizumab-treated patients were infections. At week 48, two patients in the placebo group had developed infections, while seven patients in the tocilizumab group had developed infections. This was not considered a statistically significant difference.
Results
The focuSSced study did not meet its primary endpoint, which was a change in mRSS from baseline to week 48. It did, however, demonstrate a preservation of lung function in the Tocilizumab arm. Serial spirometry plus diffusing capacity for carbon monoxide (DLCO) was measured at baseline and weeks 8, 16, 24, 36, and 48. Participants also received a baseline and week 48 high resolution CT scan (HRCT). T-tests and Spearman correlation coefficients were used to compare baseline percent predicted FVC (%pFVC) by baseline quantitative interstitial lung disease and fibrosis. Linear models were created to assess the change of %pFVC over time. Tocilizumab was shown to stabilize FVC over 48 weeks independent of baseline lung function: the least squared means of %pFVC change was -0.1% for the Tocilizumab arm and -6.3% for the placebo arm. For mild, moderate, and severe categories, the mean decline in %pFVC in the tocilizumab arm at 48 weeks was -4.1, 0.7, and 2.1 and in the placebo group was -10.0, -5.7, and -6.7, respectively. Participants in the placebo arm had worsening lung fibrosis on HRCT at 48 weeks whereas those in the Tocilizumab arm did not.
Discussion
The focuSSced trial population differed from prior landmark systemic sclerosis associated ILD studies in that they had significantly less fibrosis. The study demonstrated preservation of FVC in the Tocilizumab arm independent of baseline lung disease, emphasizing the importance of early screening and intervention. Now that treatment options for scleroderma associated ILD have grown, a research-backed, unified treatment algorithm for scleroderma associated ILD based on disease severity is needed.
Most experts agree patients with subclinical ILD and low-risk features should likely undergo close surveillance whereas those with severe ILD warrant some combination of immunomodulation, anti-fibrotic, and escalation therapies. The question remains: how to manage subclinical ILD but with high-risk features? This includes patients with elevated acute phase reactants, early progressive skin disease, anti-Scl-70 positivity — an overlapping population to the one studied in the focuSSced trial. This is where Tocilizumab may have a role – those patients with sublinical lung disease at high risk for progression who are not already on mycophenolate; however, more research would be needed to confirm this.
| F: Follow up | 48 weeks |
| R: Randomization | Yes |
| I: Intention to treat | Yes, some of original population excluded without ILD |
| S: Similar at baseline | Yes |
| B: Blinding | Yes |
| E: Equal treatment | Not discussed |
| S: Source (funding) | NIH grant, Hoffman La-Roche |
References
- Khanna D, Lin CJF, Furst DE, et al.; focuSSced investigators. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2020 Oct;8(10):963-974.
- Distler O, Highland KB, Gahlemann M, et al.; Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 2019;380:2518–2528.
- Aringer M, Riemekasten G, Relevance of immunomodulatory therapy for interstitial lung disease in systemic sclerosis, Best Practice & Research Clinical Rheumatology, 2021, 101672.
- Roofeh D, Lescoat A, Khanna D. Treatment for systemic sclerosis-associated interstitial lung disease. Curr Opin Rheumatol. 2021;33(3):240-248.
- Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med 2016;4:708–719.
