PARADISE-MI: ARNI in Acute MI
Angiotensin Receptor-Neprilysin Inhibition in Acute Myocardial Infarction.
Pfeffer MA, Claggett B, Lewis EF, et al.; PARADISE-MI Investigators and Committees.
N Engl J Med. 2021 Nov 11;385(20):1845-1855. [Full text]
Summary by Dylan Walter
In 2014 the PARADIGM-HF trial showed demonstrated that a novel combination of a neprilysin inhibitor (sacubitril) and angiotensin receptor blocker (valsartan), often referred to as ARNi, reduced both mortality and recurrent hospitalization for patients with NYHA Class II and III heart failure and reduced ejection fraction (HFrEF) compared to a common angiotensin converting enzyme inhibitor (ACEi), enalapril [1]. In acute decompensated HFrEF, sacubitril/valsartan decreased NT-proBNP more than enalapril and also seemed to decrease readmission [PIONEER-HF, 2] The benefit in symptomatic heart failure with preserved ejection fraction (HFpEF); however, was less clear [PARAGON-HF, 3]. Subsequently, sacubitril/valsartan has become a first line in the management of HFrEF in the 2022 ACA Guidelines [4].
Less is known about how distinct types of renin-angiotensin system inhibitors (RASi) may affect progression of transient left ventricular (LV) dysfunction after an acute myocardial infarction (AMI). Further, patients suffering AMI with subsequent LV dysfunction are known to be at elevated risk of developing chronic HF and cardiovascular (CV) death [5].
SAVE, AIRE and TRACE were large clinical trials that distinguished ACEis reduce CV death and development of symptomatic HF in the AMI population while VALIANT showed comparable results for the angiotensin receptor blocker (ARB), valsartan [6, 7, 8, 9]. As a result, ACEis/ARBs are recognized as standard of care internationally in LV dysfunction after AMI [10].
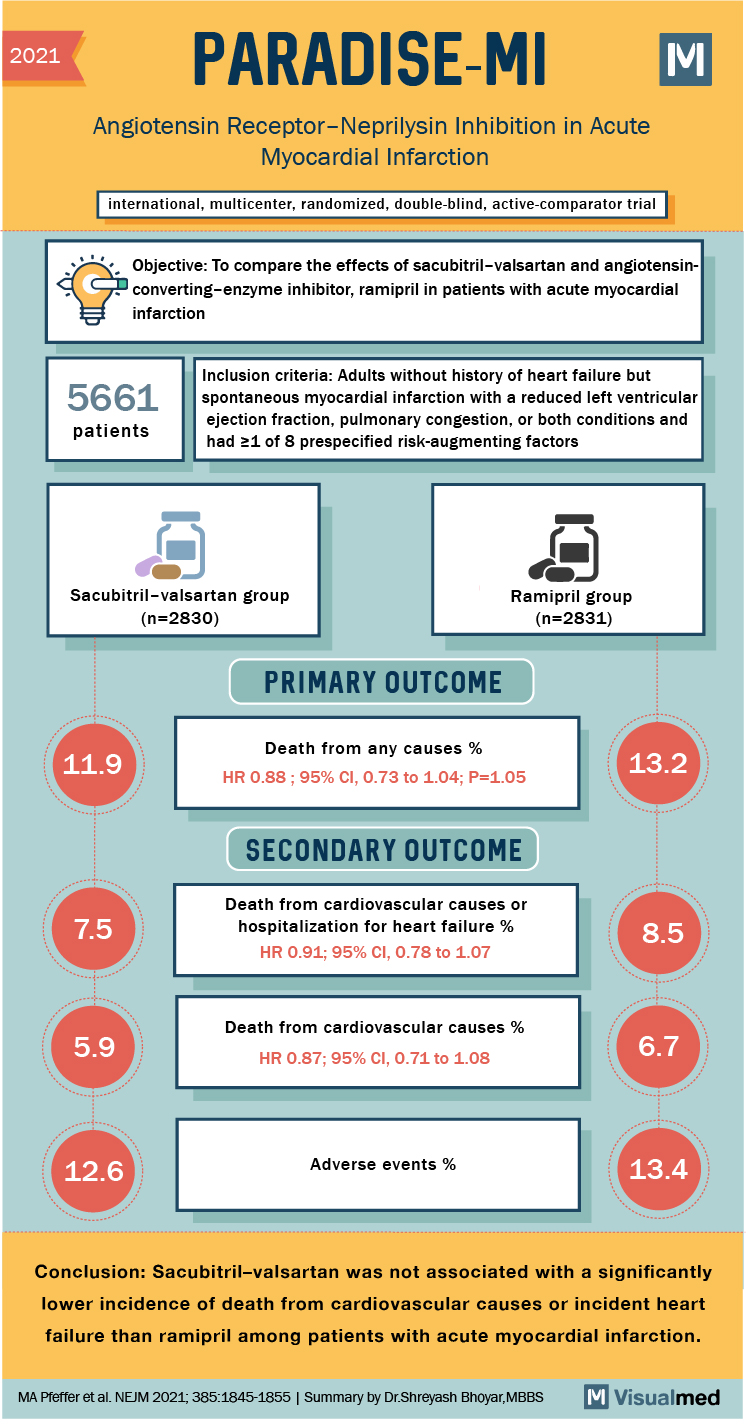
Given that sacubitril/valsartan has shown superiority compared to ACEi in the prevention of cardiovascular events and hospitalizations in the chronic and decompensated HFrEF population, it could be hypothesized that it could better prevent the development of HFrEF in those with LV dysfunction after AMI. PARADISE-MI, a double-blinded randomized controlled trial designed to address this question, comparing sacubitril/valsartan to ramipril.
Patient population and Design
Eligible patients included those that had AMI in the prior 0.5-7 days (mean 4.3) and demonstrated either reduced left ventricular ejection fraction (LVEF) < 40% (81.4%), symptomatic pulmonary congestion (54%) or both (35.5%) along with at least 1 additional risk factor associated with increased risk of HF or death (Age > 70, eGFR < 60, Diabetes, Prior MI, Atrial Fibrillation, LVEF < 30%, Killip Class III or IV and STEMI without reperfusion).
5661 patients with mean age of 64 (+/-12) were randomized to receive the trial drug (sacubitril/valsartan 24/26, Ramipril ) with an intention to titrate to 97/103 mg of sacubitril/valsartan or 5 mg BID for ramipril. The study was event-driven, targeting 708 primary endpoints for 80% power. Before initiating the study drugs, systolic blood pressure had to be at least 110 mm Hg in patients who had not taken an ACEi or ARB in the 24 hours before randomization and at least 100 mm Hg in patients who had.
Of the enrollees, 24% were female and 75% where Caucasian. 6.5% Asians and 1.3% black,. The average LVEF was 37% (+/-9). Comparison of two groups (ARNi vs. ACEi) the baseline CV risk factors showed similar percentages of prior MI (16% vs. 16%), hypertension (65% v. 65%), Diabetes (43% vs. 42%), Atrial Fibrillation (14% vs. 13%) and renal function (eGFR 72% vs. 72%). AMI characterization was also similar between study groups with the majority having STEMI (76% vs. 76%), Anterior infarction (68% vs. 68%), PCI for reperfusion (88% vs 88%) and comparable degrees of LV dysfunction (LVEF 36% vs. 37%).
Treatment groups were randomized 1:1 and compared on intention to treat basis with stratification for regression by type of MI (STEMI v. NSTEMI), Primary PCI and geographic location. A mean of 88% received reperfusion by PCI while 6% had reperfusion with thrombolysis. Dual-antiplatelet (92.2%), statin (94.9%), mineralcorticoid antagonism (MRA, 41.3%) and ACEi/ARB prior to randomization (78%) were frequently prescribed.
Among the known risk factors increasing the likelihood of HF or death, 2697 (47%), 1744 (30.8%) and 1228 (21.7%) patients had 1, 2, and 3 positive factors augmenting risk of chronic HF or CV death, respectively.
Outcomes
The composite primary outcome included cardiovascular (CV) death, 1st time hospitalization for HF or 1st time development of outpatient HF requiring diuresis. Secondary outcomes included composites of 1.) CV death or hospitalization for heart failure, 2.) hospitalization for HF or an outpatient episode of HF, 3.) CV death, nonfatal-MI or nonfatal-stroke and 4.) total hospitalizations for HF, MI or stroke. Unitary secondary outcomes included 1.) CV deaths and 2.) all-cause mortality
Results
In a median follow-up of 22 months, 338 (11.9%) composite events, described by 137 cardiovascular deaths, 164 first time hospitalizations for heart failure and 37 events of first-time symptomatic outpatient HF were identified in the sacubitril/valsartan group and were not significantly different (HR 0.90; CI 0.78 – 1.04, p =0.17) when compared to 373 (13.2%) in the ramipril group (136 CV death, 187 first HF hospitalizations, 50 first time HF outpatient).
Secondary outcomes including CV death or hospitalization for HF (HR 0.91, CI 0.78 – 1.07), hospitalization for HF or development of outpatient HF (HR 0.84, CI .70 – 1.02), CV death (HR 0.87, CI 0.71 – 1.08) and death from any cause (HR 0.88, CI 0.73 – 1.05) were not significantly different between groups. The multi-component composite outcomes of CV death, nonfatal-MI and nonfatal-stroke along with CV death and total HF hospitalizations for HF, myocardial infarction or stroke were also not significantly different.
Among participants, 1437 (50.8%) and 1606 (56.7%) were receiving target doses of the trial drugs sacubitril/valsartan and ramipril, respectively. Safety profiles of the drugs were largely comparable with 501 (17.7%) patients having discontinued sacubitril/valsartan and 517 (18.3%) having discontinued ramipril for any reason other than death. Between the sacubitril/valsartan and ramipril groups, no significant difference in overall adverse event frequency was noted (12.6% v.13.4%, p = 0.39), while the sacubitril/valsartan group had more hypotension (28% v. 21.9%; p = < 0.001) that was not clinically significant, and the ramipril group had more cough (13.1% v. 9%, p = < 0.0001). Other adverse events monitored during treatment without evidence of significant differences included angioedema (0.5% vs. 0.6%), hepatotoxicity (4.7% vs. 5.9%), hyperkalemia, (10.6% vs. 10.1%), development of cancer (3% vs. 2.5%), renal impairment (11.6% vs. 11.5%) and statin-drug interactions (3.7% vs. 4.6%).
Discussion
PARADISE-MI, a double-blind, active controlled event-driven trial comparing sacubitril/valsartan with ramipril in patients after AMI with LV dysfunction, pulmonary congestion or both did not show a significant reduction in risk for the primary composite outcome (CV death, 1st hospitalization for HF, outpatient development of HF requiring diuresis) or the various secondary outcomes.
Importantly, neither study group encountered marked adverse effects from the trial drugs suggesting excellent tolerability for both. One interesting caveat is that among patients treated with ACEi prior to randomization, no 36-hour washout period was utilized. Rather, these patients were given 2 doses of valsartan alone prior to receiving ARNi and did not develop adverse reactions, hinting that early administration of an ARNi could be safer. Similar to prior trials, the ARNI group had more hypotension, a major reason patients have to discontinue this therapy.
Importantly, since this trial was designed to elicit superiority, its results mean that ACEi and ARB remain the standard of care to reduce risk of HF development and CV death in AMI patients with LV dysfunction. While PARADISE-MI is a “negative” trisal, it is an important one in our understanding of how ARNIs are to be used clinically, for now only in established HFrEF.
| F: Follow up | Median 22 mo |
| R: Randomization | Yes |
| I: Intention to treat | Yes |
| S: Similar at baseline | Yes |
| B: Blinding | Yes |
| E: Equal treatment | Yes |
| S: Source (funding) | Novartis |
- McMurray JJ, Packer M, Desai AS, et al.; PARADIGM-HF Investigators and Committees. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004.
- Velazquez E.J., Morrow D. A., DeVore A.D., et al. for the PIONEER-HF Investigators. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med 2019; 380:539-548; [Full Text]
- Solomon SD, McMurray JJ, Anand IS, et al.; PARAGON-HF Investigators and Committees. Angiotensin – neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620
- Heidenreich PA, Bozkurt B, Aguilar D, et al.; 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022 May 3;145(18):e895-e1032.
- Van Diepen S, Chen AY, Wang TY, et al.; Influence of heart failure symptoms and ejection fraction on short- and long-term outcomes for older patients with non-ST-segment elevation myocardial infarction. Am Heart J 2014;167:267–273.e1
- Pfeffer MA, Braunwald E, Moyé LA, et al.; Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the Survival and Ventricular Enlargement Trial. The SAVE Investigators. N Engl J Med 1992;327:669–677.
- The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet 1993;342:821–828
- Kober L, Torp-Pedersen C, Carlsen JE, et al.; A clinical trial of the ACE inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. TRACE Study Group. N Engl J Med 1995;333:1670–1676
- Lewis EF, Velazquez EJ, Solomon SD, et al.; Predictors of the first heart failure hospitalization in patients who are stable survivors of myocardial infarction complicated by pulmonary congestion and/or left ventricular dysfunction: a VALIANT study. Eur Heart J 2008;29:748–756.
- O’Gara PT, Kushner FG, Ascheim DD, et al.; 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:485–510.