WISDOM – Withdrawal of ICS in COPD
“Withdrawal of Inhaled Glucocorticoids and Exacerbations of COPD“
Magnussen H, et al. for the WISDOM Investigators.
N Engl J Med. 2014 Oct 2;371(14):1285-94. doi: 10.1056/NEJMoa1407154. [Full text]
Combination inhalers, and the evidence for them in COPD is a crowded (and lucrative) space (see for example ETHOS, FULFIL, TRILOGY, TRIBUTE). We know that in COPD most therapies are for symptom management and to attempt to decrease exacerbations, but they may not have much impact on disease progression or mortality.
When WISDOM was published in 2014, long-acting muscarinic antagonists (LAMAs) were relatively new and had been shown to reduce exacerbations and LAMAs potentially have a better safety profiled than inhaled corticosteroids (ICS). With this in mind, WISDOM sought to see if patients with severe to very severe COPD on triple inhaled therapy (ICS+LABA+LABA) could be stepped down off of ICSs without increased exacerbation risk, via a non-inferiority trial, over 1 year [1].
Patient Population
A total of 2,485 adult current or former smokers (≥10 pack-years) with severe / very severe COPD (as defined by post-bronchodilator FEV1 < 50% and FEV1 <70% of FVC) that had at least 1 exacerbation in last 12 mo were recruited from 200 centers across 23 countries. Most were male (~80%) and were GOLD3 with a mean FEV1 of about 34. Key exclusion criteria were a:
- “Significant diseases other than COPD”
- History of thoracotomy
- Asthma dx on ICS
- On daytime home O2 (>1 hr / day)
- Chronic PO steroids use (>5 mg /day)
- Recent MI, HF exacerbation, or arrhythmia
- URI or recent COPD exac (inclusion delayed by 6 wk)
Study Design
There was a 6 week run-in period in which all patients were on Tiotoprium 18 mg daily, Salmeterol 50 mg BID, and Fluticasone 500 mg BID. Then 1,242 patients in the intervention arm stepped down their ICS every 6 weeks as follows: total 1000 mg -> 500 -> 200 -> 0 (placebo), such that they were off ICS at 18 weeks.
Outcomes
The primary outcome was the time to a first moderate to severe COPD exacerbation. A moderate exacerbation was defined as an increase in lower respiratory track symptoms or ≥ 2 new COPD related symptoms for at least 3 days requiring antibiotics and/or oral GCs.
Important secondary outcomes included the change in baseline FEV1 over time and symptom assessment, for example with St. George’s Respiratory Questionnaire (SGRQ). Adverse events were tracked, including the incidence of penumonia and cardiovascular events.
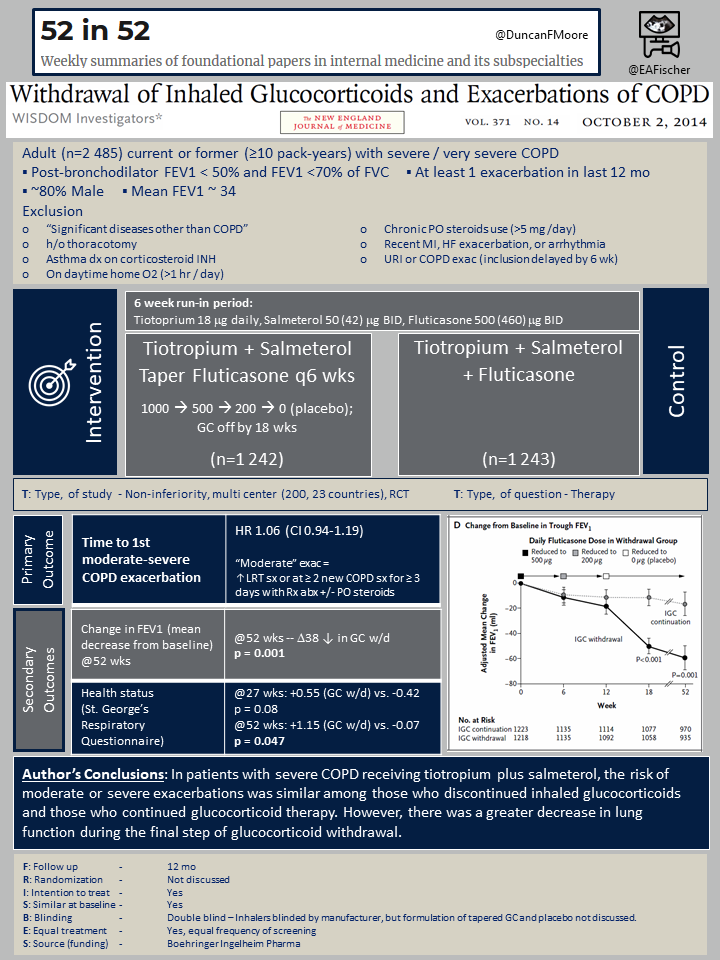
Results
Over the year long study period, the time to first moderate to severe exacerbation was similar between groups, HR 1.06 (CI 0.94-1.19, P0.35). This was the primary outcome. The secondary outcomes suggest that over that year, FEV1 and symptom control seemed to suffer. By 18 weeks the worsening of FEV1 in the ICS withdrawal group was lower statistically, and at 52 weeks it had dropped by 39 (p = 0.001). Similarly, at 52 weeks symptoms as measured by the SGRQ were worse in the ICS withdrawal group (+1.15 vs. -0.07, p = 0.047). Additionally, when looking at severe exacerbations at a year there was a trend to worsening HR 1.20 (0.98–1.48, P=0.08). There were no difference in the rates of adverse events between the groups, including similar frequency of hospitalization, death, pneumonia or cardiovascular event.
Discussion
While the study showed non-inferiority at one year regarding no change in time to the first exacerbation it has been criticized that the follow up period was too short. On average, COPD exacerbation happens every 1.3-2 years [2]; hence, the 1 year follow up period may not adequately capture the “next” exacerbation. Additionally, there seemed to be a trend in the difference in time to first severe exacerbation in the ICS withdrawal group.
The goal of inhaled therapy is to reduce exacerbation AND aid in symptom management. Though not primary outcomes, the FEV1 declined and symptoms were reported more poorly controlled in the ICS withdrawal group.
But ICS use has consequences. It has been associated with more frequent pneumonia (see IMPACT), pulmonary nontuberculous mycobacterial infection, and oral candidiasis [3]. It also should be noted that cost of triple therapy, whether in a single inhaler (e.g. Trellegy Ellipta) or a dual combo plus mono-therapy inhalers can be high. These may be factors to consider when weaning ICS.
A more recent look at de-escalation (SELECT, 2018) similarly found no increased risk of exacerbation; however, the subgroup of patients with baseline blood eosinophil counts of ≥ 300 (0.300 × 109 cells/L) had significantly greater loss of lung function and more frequent exacerbation [4]. Perhaps those with eosinophil counts below 300 could be weaned more readily. But the most recent GOLD guidelines from 2020 state that “if de-escalating ICS is considered after respiratory stability is achieved… …it should be done with caution” and suggests that pneumonia may be an appropriate indication [5].
Table 1. Pharmacotherapy, Individual Components
| Class | Drug Names (Common Trade Name) |
| Inhaled long-acting B2-agonist (LABA) | Salmeterol Formoterol Arformoterol Indacaterol |
| Inhaled long-acting anticholinergic (LAMA) | Tiotropium (Spiriva) Aclidinium Umeclidinium (Incruse Ellipta) Glycopyrrolate Revefenacin |
| Inhaled corticosteroid (ICS) | Fluticasone (Flovent) Budesonide (Pulmicort) Mometasone (Asmanex) Ciclesonide (Alvesco) Beclomethasone (Qvar) |
Table 2. Pharmacotherapy, Combination Therapy
| Class | Drug Names | Common Trade Name |
| LABA / LAMA | Tiotropium / olodaterol Umeclidinium / vilanterol Glycopyrrolate / formoterol Glycopyrrolate / indacaterol | Stiolto Respimat Anoro Ellipta Bevespi Aerosphere Utibron Neohaler |
| LABA / ICS | Salmeterol / fluticasone propionate Formoterol / budesonide Vilanterol / fluticasone furoate | Advair, AirDuo RespiClick Symbicort Breo Ellipta |
| LABA / LAMA / ICS | Umeclidinium / vilanterol / fluticasone furoate | Trelegy Ellipta |
- Reilly JJ. Stepping down therapy in COPD. N Engl J Med. 2014 Oct 2;371(14):1340-1. doi: 10.1056/NEJMe1409219.
- Cosio M, Baraldo S. Inhaled Glucocorticoids and COPD Exacerbations. N Engl J Med 2015; 372:92-94. doi: 10.1056/NEJMc1413308
- Labaki WW, Rosenberg SR. Chronic Obstructive Pulmonary Disease. Ann Intern Med. 2020 Aug 4;173(3):ITC17-ITC32.
- Chapman KR, Hurst JR, Frent SM, et al. Long-Term Triple Therapy De-escalation to Indacaterol/Glycopyrronium in Patients with Chronic Obstructive Pulmonary Disease (SUNSET): A Randomized, Double-Blind, Triple-Dummy Clinical Trial. Am J Respir Crit Care Med. 2018 Aug 1;198(3):329-339.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2020 Report.
