ProCESS
A Randomized Trial of Protocol-Based Care for Early Septic Shock
ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al.
N Engl J Med. 2014 May 1;370(18):1683-93. [Full Text]
Summary by Samuel B. Pach
Unquestionably the 2001 Rivers et al. that introduced the 6 hour protocol, or “early goal-directed therapy” (EGDT), was a landmark in sepsis management [1, 52in52 Rivers]. While the intervention showed a mortality benefit, individual elements were shown less beneficial, unreliable (i.e. CVP) or even harmful (Hgb to 10) in separate studies but were essentially entrenched (i.e. in sepsis guidelines) given the bundles’ demonstrated “effect”.
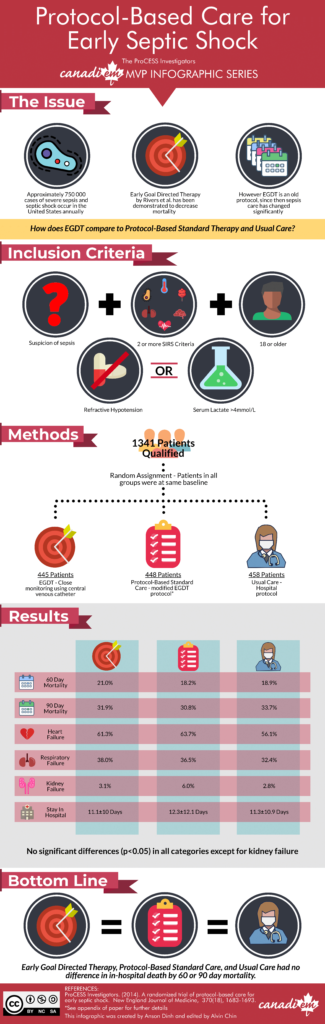
The 2014 Protocolized Care for Early Septic Shock (ProCESS) aimed to re-evaluate protocol and looked at a protocol that had been revamped with knowledge gained since the Rivers trial. This was compared to the traditional EGDT protocol as well as to usual care. The randomized study took place in 31 emergency departments in the United States.
Methods
The primary end point was 60 day in hospital mortality. Secondary outcomes were longer term mortality and the need for organ support.
Criteria for patient selection included: those meeting two or more systemic inflammatory response syndrome (SIRS) criteria, those with refractory hypotension (SBP < 90 mmHg or requiring vasopressor support after initial fluid challenge) or serum lactate of ≥ 4 mmol per liter or higher.
Exclusion criteria as as follows:
- Acute cerebral vascular event
- ACS
- Acute pulmonary edema
- Status asthmaticus
- “Major” cardiac arrhythmia
- Active GIB
- Seizure
- Drug overdose
- Burn
- Trauma
- Need for immediate surgery
- CD4 count <50
- Advanced directive that would restrict implementation of the protocol
- Contraindication for central venous catheter
- “High likelihood” of blood transfusion refusal (e.g. Jehovah’s Witness)
- Futility of resuscitation as deemed by treating physician
- Enrolment in another interventional study
- Known pregnancy
- Transfer as inpatient from other hospital
Protocol based EGDT (designed to mimic the Rivers study) included, placement of central venous catheter and aggressive hemodynamic goals (CVP, SVCO2, n=439). The novel protocol design (n=446) was based on a review of the literature, two surveys of emergency physicians and intensivists across the world, and feedback from investigators. It did not require central intravenous catheter placement unless peripheral access was not sufficient. Patients in the usual care group (n=458) received care at the discretion on bedside providers.
The clinical teams and investigators at each individual site were made unaware of study group outcomes until the data was locked in December 2013.
Results
Notably, all groups in the study received on average >2 liters of fluid prior to randomization and >75% received antibiotics prior to randomization.
After randomization, initial fluid resuscitation was noted to differ among the three groups. Over the first 6 hours, 2.8 liters were given in the protocol based EGDT group, 3.3 liters in the protocol based standard group, and 2.3 liters in the usual-care group (P<0.001). Patients in the two protocol based groups were more likely to receive vasopressors (54.9% in the protocol based EGDT group and 52.2% in the protocol based standard therapy group vs 44.1% in the usual care group p=0.003). The use of antibiotics, glucocorticoids, and activated protein C was similar across the three groups.
At 60 days there were 92 deaths in protocol based EGDT group (21%), 81 in protocol-based standard therapy group (18.2%), and 86 in the usual-care group (18.9%) (RR with protocol-based therapy vs. usual care, 1.04; 95% CI 0.82 to 1.31; P = 0.83; RR with protocol-based EGDT vs. novel protocol-based standard therapy, 1.15; 95% CI 0.88 to 1.51; P = 0.31). There were no significant differences in 90-day mortality, 1-year mortality, or the need for organ support.
Incidence of acute renal failure and need for renal replacement therapy was higher in the EGDT protocol based therapy group than in the other two groups (6.0% in the protocol-based standard therapy group vs 3.1% in the protocol based EGDT group and 2.8% in the usual-care group, P =0.04). There were more ICU admissions in the EGDT group: 91.3% vs. 85.4%, vs. 86.2% usual care. No significant differences were noted in the incidence/duration of cardiovascular or respiratory failure. Additionally, no significant differences were noted in the length of stay in the hospital or disposition upon discharge.
Discussion
This study demonstrated that the traditional protocol based care for sepsis (standard or EGDT) resulted in higher use of central venous catheterization, intravenous fluids, vasoactive agents, and blood transfusions. The comparisons groups, which required less interventions, was non-inferior in regards to mortality. Of note, the patients used in River et al were slightly older and had higher rates of preexisting heart and liver failure. Additionally these patients had higher serum lactate levels. Despite this, no benefit for standard EGDT protocol based care was demonstrated even when study researchers analyzed only the sickest one third of patients (highest lactate levels and highest APACHE II scores).
Limitations identified by the study authors included: difficulty confirming full adherence to resuscitation protocols, patient care prior to randomization was quite variable and this certainly could have impacted outcomes in a disease state as varied as sepsis. Additionally, there was not sufficient power to evaluate whether or not specific care models were more effective within specific subgroups, and finally mortality can vary significantly based on site specific practices regarding withdrawal of care.
One major consideration is that the protocol based care made widespread by Rivers et al and was standard of care at the time influenced care such that providers were effectively implementing major portions of it regardless of group assignment (recall that volume and antibiotics given to all before randomization).
Bottom line
In this multicenter trial conducted in tertiary care emergency departments across the United States, protocol based resuscitation compared to non-protocol based “usual care” in patients with septic shock did not effect outcomes. Additionally, there was no significant benefit to warrant mandating the use of central venous catheterization or central hemodynamic monitoring.
More recently, there have been two other large RCTs of EGDT versus usual care and/or protocols that used some of the EGDT targets: ARISE (2014, Australia), and ProMISe (2015, UK). In general terms, EGDT provided no mortality benefit compared to usual care. Prospectively, the authors of these trials and ProCESS planned a meta-analysis – the 2017 PRISM study – which concluded that “EGDT did not result in better outcomes than usual care and was associated with higher hospitalization costs across a broad range of patient and hospital characteristics.” Despite patients in the Rivers trial being sicker than those of ProCESS/ARISE/ProMISe, it was not found in the subgroup analysis of PRISM that EGDT was more beneficial in sicker patients. Overall, the PRISM authors noted that “it remains possible that general advances in the provision of care for sepsis and septic shock, to the benefit of all patients, explain part or all of the difference in findings between the trial by Rivers et al. and the more recent trials.”
Source/Funding: National Institute of General Medical Sciences; ProCESS
- Rivers E, Nguyen B, Havstad S, et al. ; Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001 Nov 8;345(19):1368-77. doi: 10.1056/NEJMoa010307. PMID: 11794169.