VERT
“Effects of Risedronate Treatment on Vertebral and Nonvertebral Fractures in Women With Postmenopausal Osteoporosis”
by Harris ST, Watts NB, Genant HK, et. al. affiliated with the Vertebral Efficacy With Risedronate Therapy (VERT) Study Group
JAMA. 1999 Oct 13;282(14):1344-52. [Full Text]
Summary by Natalie Dapas
Menopause leads to higher risk of bone fracture because of an increase in bone turnover and a decrease in bone mass associated with lower levels of estrogen. Risedronate is a bisphosphonate used to treat metabolic bone diseases that has also been shown to prevent early postmenopausal bone loss. This randomized, double-blind, placebo-controlled trial examined the ability of risedronate to decrease fracture risk in women with postmenopausal osteoporosis.
Patient Population
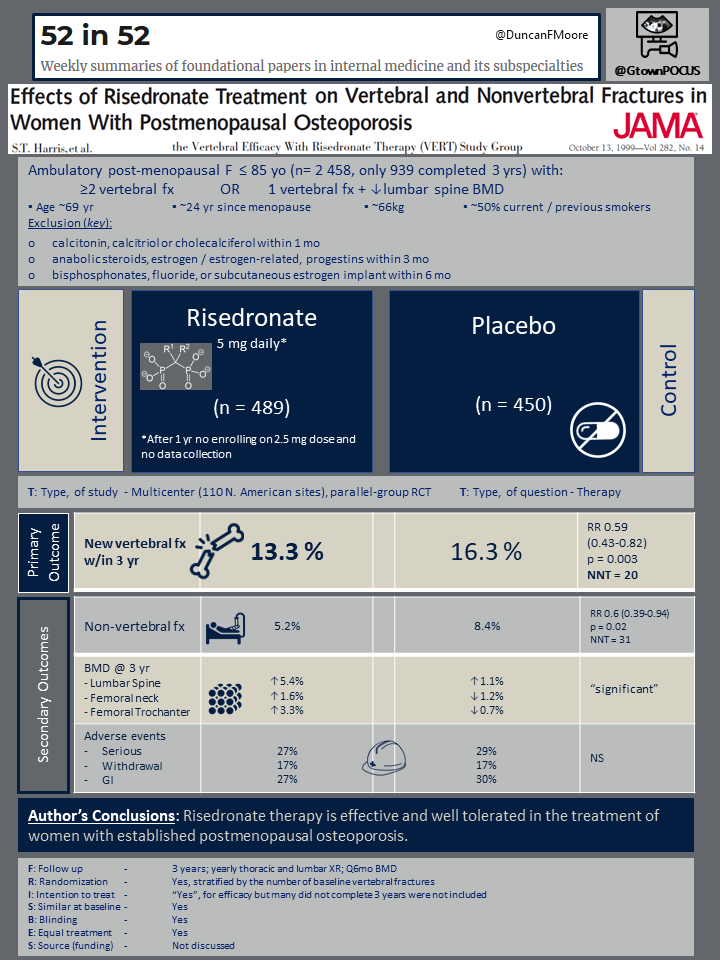
This study included 2458 ambulatory women who were younger than ≤ 85 and had experienced surgical or natural menopause at least five years prior to enrollment. Subjects had either ≥2 vertebral fractures as determined by radiology or 1 vertebral fracture AND decreased lumbar-spine BMD. Baseline time from menopause was 24 years.
Exclusion criteria were as follows:
- calcitonin, calcitriol or cholecalciferol within 1 mo
- anabolic steroids, estrogen / estrogen-related, progestins within 3 mo
- bisphosphonates, fluoride, or subcutaneous estrogen implant within 6 mo
Outcomes
Participants were randomly divided to receive oral risedronate (2.5 or 5mg/d) or placebo for 3years. Patients were similar at baseline. The primary outcome was new vertebral fracture incidence at 3 years. Other outcomes included new non-vertebral fracture incidence, and bone mineral density change at 3 years. Baseline and annual thoracic and lumbar radiographs were obtained. Bone mineral density was obtained at the beginning of the study as well as at six-month intervals. Finally, adverse events were monitored and reported.
Results
After the first year, the risedronate 2.5 mg/d arm was terminated as the author’s reported from published data found 2.5-mg to be inferior to the 5-mg dose. 60% of the risedronate 5 mg/d arm and 55% of the placebo arm finished the 3 year treatment. The risedronate 5 mg/d arm (489 subjects) had reduced vertebral fractures compared to the placebo arm (450 subjects) over three years, 13.3% vs. 16.3% (RR 0.59, 95% CI 0.43-0.82, P = 0.003, NNT = 20). Similarly, the cumulative incidence of nonvertebral fractures was decreased over 3 years, 5.2% vs. 8.4% (RR 0.6, 95% CI 0.39-0.94, P = 0.02, NNT = 31).
Compared to placebo, there was an improvement in bone mineral density at the lumbar spine (5.4% vs 1.1%), femoral trochanter (3.3% vs -0.7%), femoral neck (1.6% vs -1.2%), and midshaft of the radius (0.2% vs-1.4%). There were no differences in the rates of serious adverse events, withdrawals from treatment, or GI side effects.
Discussion
Overall, this large, well-designed randomized controlled trial illustrated clear benefit of risedronate in decreasing fracture risk with a safety profile comparable to that of placebo. Of note, the study included patients with gastrointestinal disorders and there was no difference in incidence of adverse events related to the upper GI tract in the treatment arm and placebo arm (though there was a moderately high withdrawal rate, risedronate or placebo). Of those subjects who withdrew from the trial, 19.6% of the placebo arm had incident vertebral fractures compared to 10.6% of the risedronate 5mg/d arm, which could have decreased the treatment effect.
Another weakness is the modification of the trial protocol to eliminate the risedronate 2.5mg treatment arm after 1 year of study. Although this arm demonstrated a reduction in vertebral fracture at 1 year relative to placebo (p = 0.02), its elimination raises suspicion that the pre-specified analyses were not yielding the anticipated results during the interim analysis and thus the less-impressive treatment arm was discarded.
But regardless, based on VERT and other trials, oral bisphosphonate therapy is the standard of care both for treatment and prevention of osteoporosis.
See also original 52in52 post by Duncan Moore.
