SPYRAL HTN-OFF MED Pivotal
Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial.
Böhm M, Kario K, Kandzari DE, et al.; SPYRAL HTN-OFF MED Pivotal Investigators.
Lancet. 2020 May 2;395(10234):1444-1451. [Full Text]
Many patients have resistant hypertension (HTN), blood pressure that remains uncontrolled despite multiple anti-hypertensives. Early proof-of-concept and safety studies showed catheter-based renal denervation were promising in resistant HTN. Then the 2014 randomized, blinded, sham-controlled SYMPLICTY HTN-3 trial showed reduction from baseline in blood pressure in both the renal denervation and sham control groups, without a significant difference between the two groups.
These observations compared to prior results and that the sham group had such a robust response were a surprise. Post-hoc analysis looking for confounding factors found frequent failures to complete the actual ablation procedural and high medication variability (after randomization 39% of patients underwent medication changes) [1].
The SPYRAL HTN-OFF Med Pivotal Trial was designed to control for those confounding factors. It was a international multicenter, prospective, single-blind, randomized, sham-controlled trial with patients enrolled off of all antihypertensive medications.
Patients
Adults (n=331) with uncontrolled hypertension that were not on any antihypertensives (or able to come of medication) were randomized to renal denervation (n = 166) versus sham (n = 165). Patients must have an office systolic blood pressure (SBP) had to be ≥150 and <180 mm Hg, office diastolic blood pressure (DBP) ≥90 mm Hg or systolic 24-hour mean ambulatory blood pressure ≥140 and <170 mm Hg. The mean age was 52 years, 35% were female and only 3.6% had diabetes.
Those that were excluded had Ineligible renal artery anatomy, CKD 3B or worse (eFGR <45), diabetics with A1c >8.0% or had a known cause of secondary HTN.
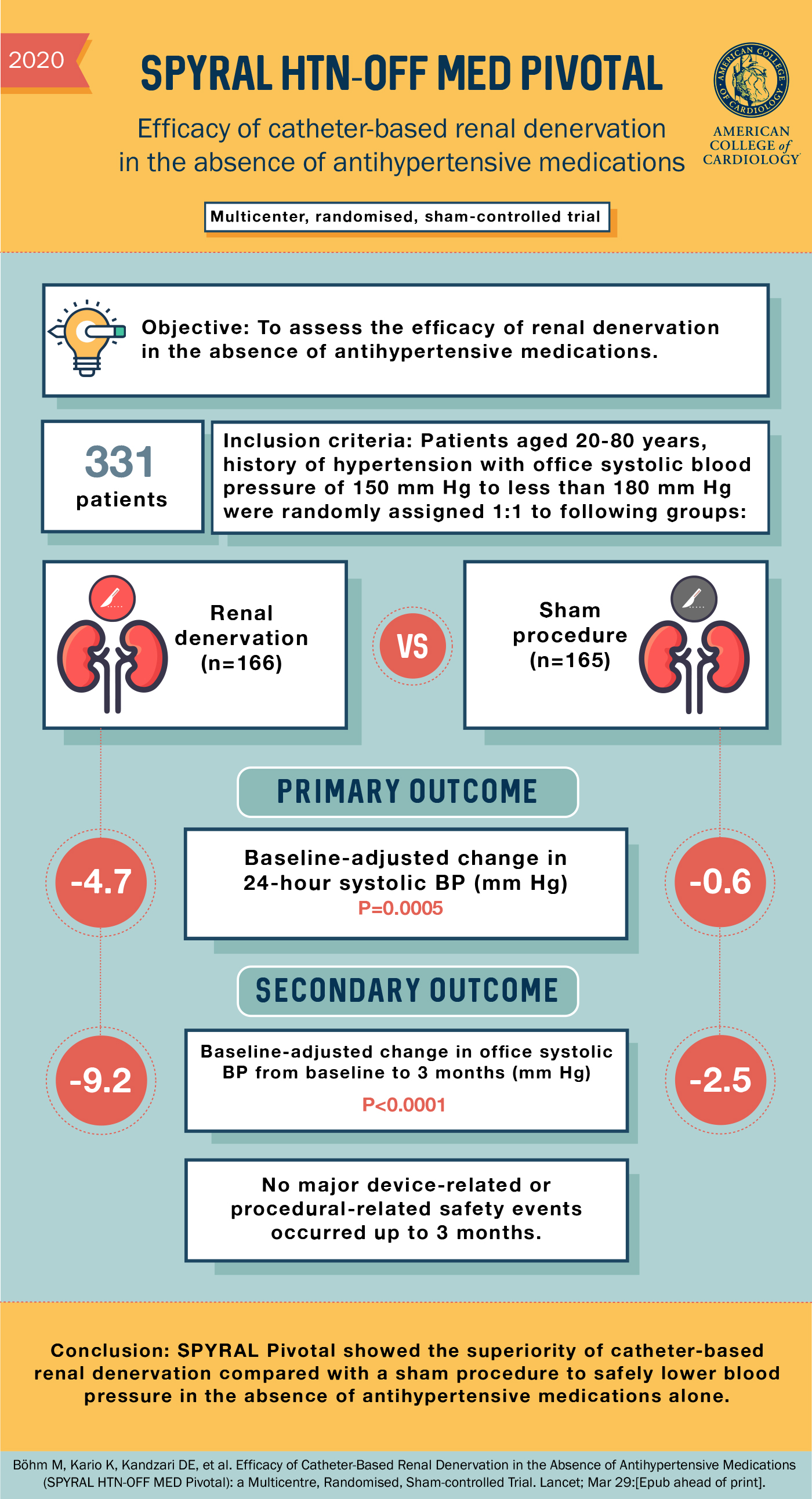
Results
The co-primary outcome of change in 24-hour SBP at 3 months, was -4.7 mm Hg in the renal denervation group compared with -0.6 mm Hg in the sham group (p < 0.001). The co-primary outcome of change in 24-hour DBP at 3 months, was -3.7 mm Hg in the renal denervation group compared with -0.8 mm Hg in the sham group (p < 0.001).
The co-primary outcome, change in office SBP at 3 months, was -9.2 mm Hg in the renal denervation group compared with -2.5 mm Hg in the sham group (p < 0.001). The co-primary outcome, change in office DBP at 3 months, was -5.1 mm Hg in the renal denervation group compared with -1.0 mm Hg in the sham group (p < 0.001).
Secondary outcomes included the number of patients placed on antihypertensive therapy due to SBP >180 mm Hg or for safety reasons was 9.6% in the renal denervation group compared with 17.0% of the sham group (p = 0.049)
Discussion
Among patients with uncontrolled HTN, renal artery denervation compared with sham was associated with a significant reduction in SBP and DBP at 3 months. The three month follow up design could have actually underestimated the true treatment effect of renal denervation. Many patients in the sham group had to resume medications under protocol defined escape criteria either because of SBPs > 180 or blood pressure related symptoms or complications. This could have decreased the difference between groups. What remains to be seen is the results of long term follow-up and results from a more reflective, real world study in which patients remain on antihypertensive medications. This is currently in process. For now this remains promising but not ready for routine use.
References
- Kandzari DE, Bhatt DL, Brar S, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015 Jan 21;36(4):219-27.
