REDUCE-IT
Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia
D. L. Bhatt, et al. for the REDUCE-IT Investigators
N Engl J Med. 2019 Jan 3;380(1):11-22. doi: 10.1056/NEJMoa1812792. [Full Text]
Elevated triglyceride (TG) levels are known to be a marker for ischemic events, and while medications such as niacin and fibrates reduce TG levels they have not been shown to decrease ischemic events. Similarly Omega acids have had little effect on such outcomes [1,2].
Icosapent ethyl is a purified form of eicosapentaenoic acid, a type of omega-3 fatty acid. Prior studies that laid the groundwork for REDUCE-IT included the MARINE [3] and ANCHOR [4] studies, which showed that twelve-week treatment with icosapent ethyl reduced triglyceride levels by 33.1 and 21.5% respectively [5]. The Japan EPA Lipid Intervention Study (JELIS), was a prospective, unblinded / open-label study of 18,645 patients that found that the risk of composite events, which included sudden cardiac death, fatal and non-fatal myocardial infarction, unstable angina, and revascularization were 19% lower in patients who received Icosapent ethyl and statin therapy vs statin therapy alone [6].
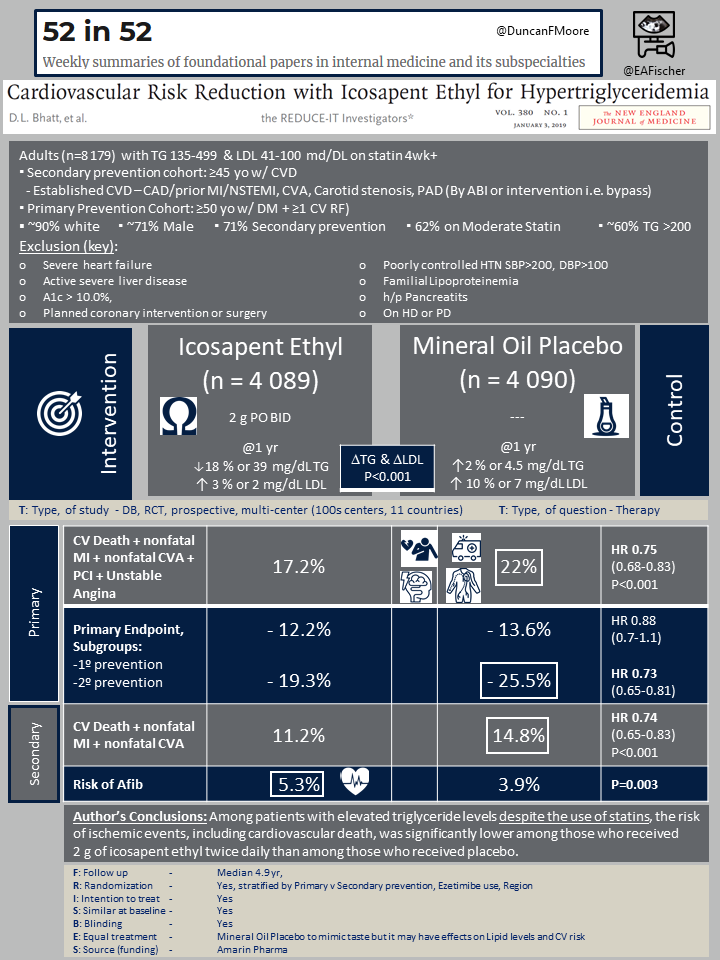
REDUCE-IT was a international multicenter, randomized, double-blind, placebo-controlled trial aimed to examine if icosapent ethyl would reduce cardiovascular events in comparison to placebo. One major consideration is that mineral oil was used as the placebo to mimic the omega taste even though mineral oil is thought to confer increased CV risk.
Patient population
8179 patients who had cardiovascular disease or diabetes and at least one additional risk factor who were currently on statin therapy were enrolled. Patients also had residual hypertriglyceridemia, with a fasting triglyceride level between 150-499 mg/dl. Exclusion criteria included those with severe heart failure, active severe liver disease, an A1c > 10.0%, and those with planned coronary intervention or surgery. The median age was 64 years, with 28.8% of patients being female, and 90% of patients being white.
Patients were randomized to either receive icosapent ethyl 2 g twice daily or mineral oil placebo. Randomization was stratified to a primary-prevention cohort (capped at 30% of patients) or secondary-prevention cohort.
Outcomes
The primary outcome was a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina. Median follow-up period was 4.9 years.
The key secondary end point was a composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. Additional endpoints included cardiovascular death, myocardial infarction, and hospitalization for unstable angina.
Results
Most patients were male (~71%), and most were recruited in the secondary prevention arm (~70%).
17.2% of patients in the icosapent ethyl group experienced primary end-point events (composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina), compared with 22.0% of patients in the placebo group (HR, 0.75; 95% confidence interval, 0.68 to 0.83; P<0.001). The NNT to prevent a primary outcome in one patient was 21.
11.2% of patients in the icosapent ethyl group experienced key secondary endpoint events, compared with 14.8% of the patients in the placebo group (HR, 0.74; 95% CI, 0.65 to 0.83; P<0.001). The NNT to prevent one key secondary end-point event was 28.
The icosapent ethyl group had a higher risk of atrial fibrillation (5.3% vs. 3.9%, p<0.001) and equal frequency of pneumonia (2.6% vs. 2.9%, p=0.42).
Discussion
Among white patients with residual hypertriglyceridemia on a statin and known cardiovascular disease or risk factors for cardiovascular disease, concomitant use of icosapent ethyl was shown to lower the risk of major cardiovascular events such as composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, coronary revascularization, or unstable angina compared to a mineral oil placebo (NNT 21).
Though this trial did demonstrate a significant decrease in the risk of other secondary endpoints including cardiovascular death (4.3% vs. 5.2%; HR, 0.80; 95% CI, 0.66 to 0.98; P=0.03), myocardial infarction (6.1% vs. 8.7%; HR, 0.69; 95% CI, 0.58 to 0.81; P<.001), and hospitalization for unstable angina death (2.6% vs. 3.8%; HR, 0.68; 95% CI, 0.66 to 0.98; P=0.03), it should be noted the absolute risk reduction between these is modest.
The findings are promising but it should be noted that the beneficial effects seem to be out of proportion to the amount TGs were lowered or the anti-inflammatory effects seen in CRP reduction [1]. In light of numerous prior negative fish oil studies [2], the unknown impact of mineral oil use as a placebo, and that icosapent ethyl may currently be cost-prohibitive (wholesale price of $334.30 for a month supply [7]) it is unlikely that these results change current management of hypertriglyceridemia without future confirmation.
Summary by Abra Guo
| F: Follow up | Median 4.9 yr |
| R: Randomization | Yes, stratified by Primary v Secondary prevention, Ezetimibe use, Region |
| I: Intention to treat | Yes |
| S: Similar at baseline | Yes |
| B: Blinding | Yes |
| E: Equal treatment | Mineral Oil Placebo to mimic taste but it may have effects on Lipid levels and CV risk |
| S: Source (funding) | Amarin Pharma |
- Kastelein JJP, Stroes ESG. FISHing for the Miracle of Eicosapentaenoic Acid. N Engl J Med. 2019 Jan 3;380(1):89-90. doi: 10.1056/NEJMe1814004. Epub 2018 Nov 16.
- Aung T, Halsey J, Kromhout D, et al.; Omega-3 Treatment Trialists’ Collaboration. Associations of Omega-3 Fatty Acid Supplement Use With Cardiovascular Disease Risks: Meta-analysis of 10 Trials Involving 77 917 Individuals. JAMA Cardiol. 2018 Mar 1;3(3):225-234. doi: 10.1001/jamacardio.2017.5205.
- Bays HE, Ballantyne CM, Kastelein JJ, et al.; Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol. 2011 Sep 1;108(5):682-90.
- Ballantyne CM, Bays HE, Kastelein JJ, Stein E, Isaacsohn JL, Braeckman RA, Soni PN. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012 Oct 1;110(7):984-92.
- Jia X, Koh S, Al Rifai M, Blumenthal RS, Virani SS. Spotlight on Icosapent Ethyl for Cardiovascular Risk Reduction: Evidence to Date. Vasc Health Risk Manag. 2020;16:1-10. 2020 Jan 9. doi:10.2147/VHRM.S210149
- Yokoyama M, Origasa H, Matsuzaki M, et al.; Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007 Mar 31;369(9567):1090-8. doi: 10.1016/S0140-6736(07)60527-3.
- Jia X, Akeryod J, Nasir K, et al. Eligibility and Cost for Icosapent Ethyl Based on the REDUCE-IT Trial. Circulation. 2020;139: 1341-1343. 2019 Mar 5.
