DAPA-HF
Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction
McMurray JJV, Solomon SD, Inzucchi SE, et al.; DAPA-HF Trial Committees and Investigators.
N Engl J Med. 2019 Nov 21;381(21):1995-2008. doi: 10.1056/NEJMoa1911303. [Full Text]
Summary by Nayrana Griffith, MD, MBA
Heart failure with reduced ejection fraction (HFrEF) accounts for about half of all cases of heart failure in the United States. Data from several clinical trials have supported class I recommendations for medication therapy in HFrEF. Currently, guideline directed therapy includes the use of beta blockers, ARNI, aldosterone inhibitors, and hydralazine and isosorbide dinitrate.
Large clinical trials involving patients with type 2 diabetes have shown that sodium-glucose cotransporter 2 (SGLT2) inhibitors reduce the risk of hospitalization for heart failure in patients with type II diabetes. Given the mechanism of SGLT2 inhibitors (ie. diuretic effect, effects on myocardial metabolism, preservation of renal function), the authors of DAPA-HF hypothesized that patients with baseline heart failure without diabetes could also benefit from these medications.
DAPA-HF prospectively evaluated the efficacy and safety of the SGLT2 inhibitor dapagliflozin in patients with heart failure and reduced ejection fraction, regardless of the presence or absence of diabetes.
Patients:
This study was conducted from February of 2017 through August of 2018. A total of 4744 patients were randomly assigned to either dapagliflozin or matching placebo at 410 centers in 20 countries. During the screening period, 42% of patients in each trial group had a history of type 2 diabetes, and an additional 3% of the patients in each group received a new diagnosis of diabetes.
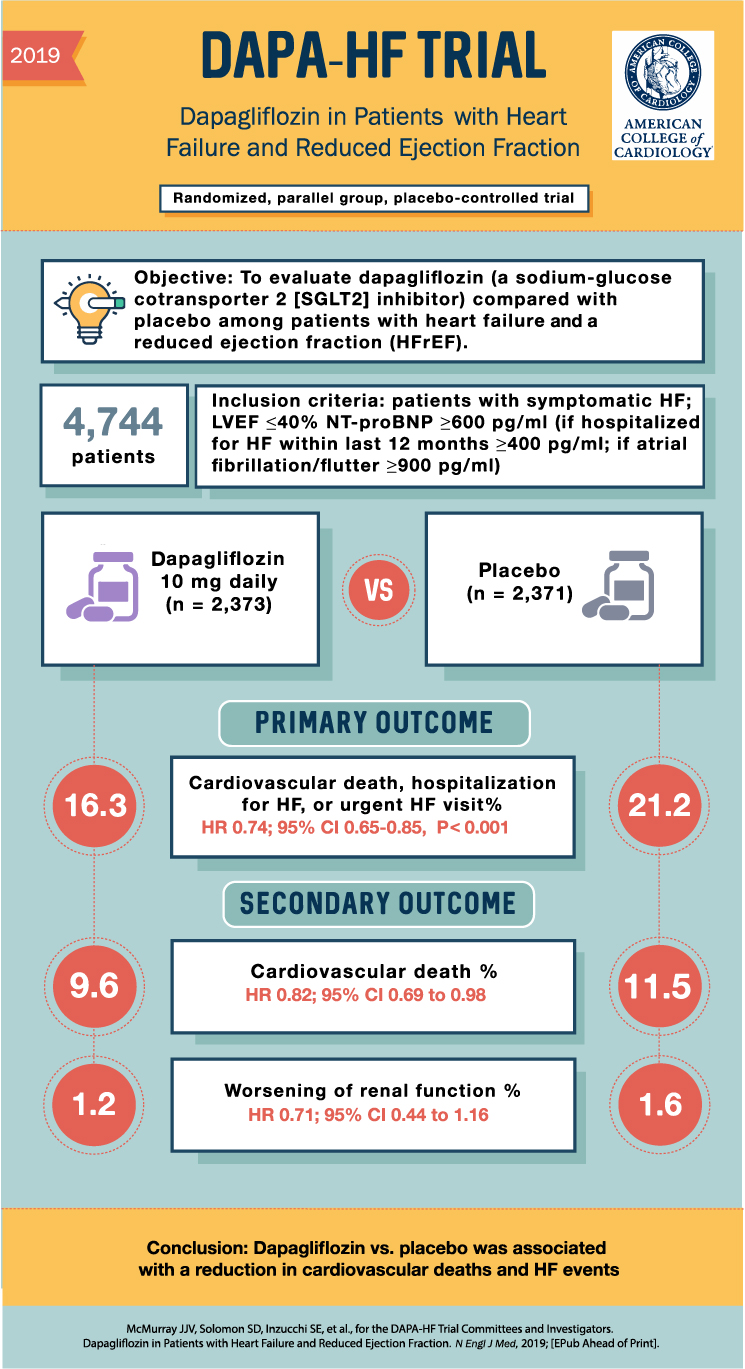
Results
Primary Outcome
The primary outcome was a composite of worsening heart failure or death from cardiovascular causes. 386 patients (16.3%) in the intervention group had a primary event compared to 502 patients (21.2%) in the placebo group (hazard ratio 0.74; 95% CI 0.65-085; P<0.001).
Secondary Outcomes
There was a lower incidence of the composite of hospitalizations for heart failure or death from cardiovascular causes in the dapagliflozin group than in the placebo group (hazard ratio, 0.75; 95% CI, 0.65 to 0.85; P<0.001). Furthermore, the increase in the total symptom score on the KCCQ (ie. indicating fewer symptoms) was greater in the dapagliflozin group than in the placebo group. Lastly, 11.6% of patients in the intervention group compared to 13.9% in the placebo group died from any cause. There was no different between the treatment groups in incidence of worsening renal function.
Safety
Serious adverse events related to volume depletion occurred in 1.2% in the intervention group and in 1.7% in the placebo group (P=0.23). Discontinuation of treatment due to adverse events rarely occurred..
Discussion
In this randomized, placebo-controlled trial involving patients with heart failure with reduced ejection fraction, the SGLT2-inhibitor dapagliflozin decreased rates of cardiovascular death and worsening heart failure. Furthermore, the use of dapagliflozin also resulted in fewer symptoms of heart failure (as measured on the KCCQ), as well as all-cause mortality. A subgroup analysis showed that the SGLT2-inhibitor was as effective in the patients with type II diabetes as well as without diabetes.
The findings of this study suggests that SGLT2-inhibitors in patients with heart failure may provide cardiovascular benefits other than lowering glucose. Thus, the authors suggest that their findings potentially extend the therapeutic role of dapagliflozin in heart failure beyond patients with diabetes.
The US FDA has approved Dapagliflozin for use in patients with HFrEF with or without diabetes, This year (2021) the American College of Cardiology (ACC) has updated guideline directed medical therapy (GDMT) for HFrEF [1]. First line medical therapy is an ARNI/ACEI/ARB with ARNI preferred based on trials such as PARADIGM-HF. This is to be combined with evidenced based beta-blockers like metoprolol succinate or carvedilol. The SGLT2 inhibitors are to be considered as add-on therapy in HFrEF with or without diabetes and NYHA class II–IV HF. If dapagliflozin is to be considered, the eGFR should be >30 mL/min/1.73 m2. For empagliflozin, the eGFR needs to be >20 mL/min/1.73 m2.
Bottom Line
The results of DAPA-HF confirm the benefit of SGLT-2 inhibitors in patients with heart failure with reduced ejection fraction and provide initial evidence of their benefits in patients without diabetes. Based on 2021 updated ACC guidelines, one may consider initiating SGLT-2 inhibitors in patients with HFrEF regardless of the presence of diabetes as add on to first line ARNI/ACEI/ARB and evidenced based beta-blockers.
References
- Writing Committee, Maddox TM, Januzzi JL Jr, Allen LA, et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021 Feb 16;77(6):772-810.
- McMurray JJ, Packer M, Desai AS; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014 Sep 11;371(11):993-1004.
- Neal B, Perkovic V, Mahaffey KW, et al.; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017 Aug 17;377(7):644-657.
- Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015 Nov 26;373(22):2117-28.
- Wiviott SD, Raz I, Bonaca MP; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019 Jan 24;380(4):347-357.

