ATTIRE – Albumin to Prevent Infection in Cirrhosis
A Randomized Trial of Albumin Infusions in Hospitalized Patients with Cirrhosis. N Engl J Med. 2021 Mar 4;384(9):808-817.
China L, Freemantle N, Forrest E, et al.
Summary by Nha-Han Pham
Patients with decompensated cirrhosis are more susceptible to infections in part owing to increased systemic inflammation, which can contribute to kidney failure and death. Albumin infusions can be utilized to attempt to compensate for associated peripheral vasodilation. Guidelines recommend use of albumin after large volume paracentesis, in patients with spontaneous bacterial peritonitis, and for hepatorenal syndrome; however, there has been conflicting results from studies examining albumin’s general ability to prevent infections in patients with cirrhosis.
A observational study found an association of low serum albumin to increased the risk of death in patients with cirrhosis with infection [1]. Albumin infusions that increased serum albumin levels to >3 g/dL are associated with reduced systemic inflammation [2] and reduced incidence of nosocomial infection among patients with decompensated cirrhosis hospitalized with nonspontaneous bacterial peritonitis [3]. The ATTIRE (Albumin to Prevent Infection in Chronic Liver Failure) trial was a prospective, multicenter, and randomized trial conducted to evaluate if targeting an increase in serum albumin to 3 g/dL or more with daily infusions of 20% albumin would reduce the incidences of infection, kidney complications, and death in hospitalized patients with decompensated cirrhosis.
Patient population and Design
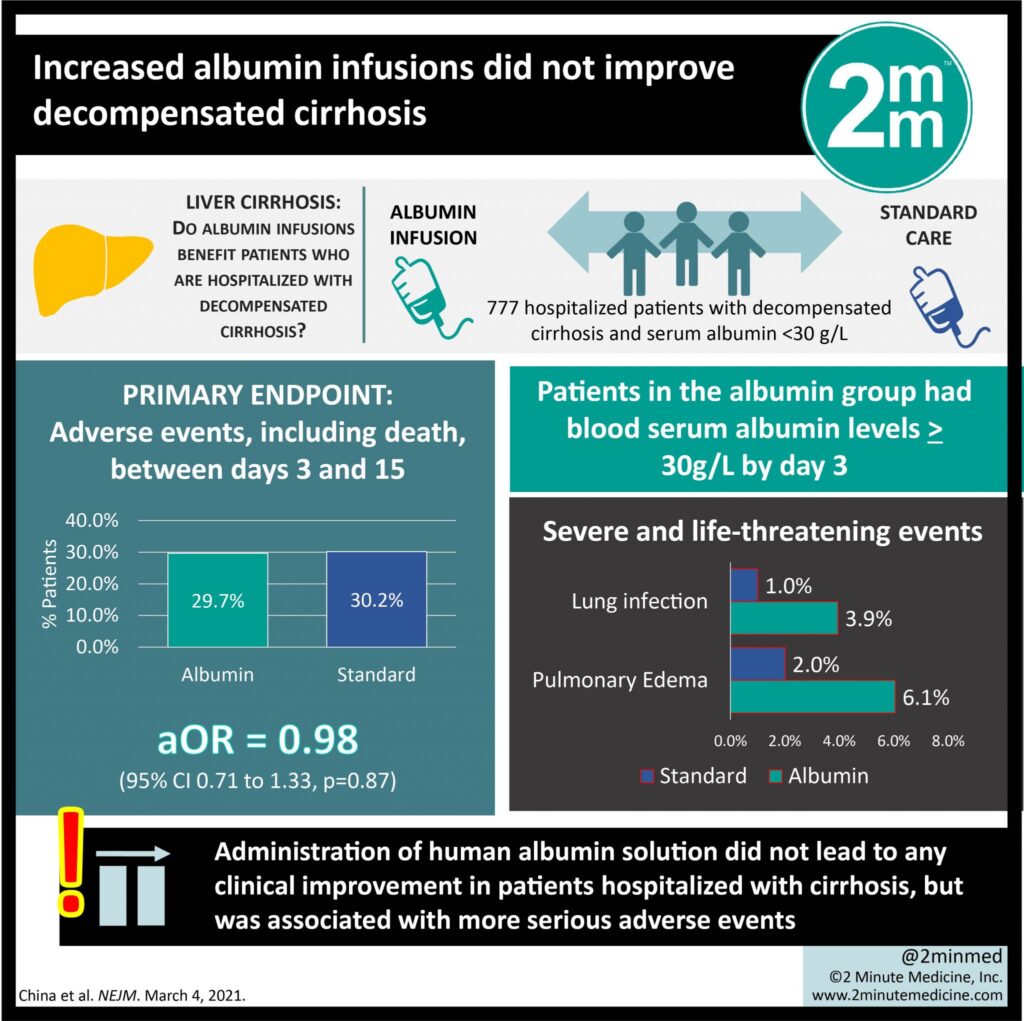
The study was conducted from January 2016 to June 2019 including patients from 35 different hospitals across England, Scott, and Wales. Inpatients that were at least 18 years old with decompensated cirrhosis and had a serum albumin of < 3 g/dL within the first 72 hours of admission. These patients also needed to have a predictive hospital stay of 5 days or longer. Patients were excluded if they had advanced hepatocellular carcinoma with left expectancy less than 8 weeks.
All the patients had serum albumin of less than 30 grams per liter and they were randomized to either daily 20% human albumin solution (infused at 100ml/hr) to achieve a serum albumin of 3 g/dL or standard of care. Daily albumin infusions continued for a maximum of 14 days or date of discharge, whichever came first.
Of note 20 % albumin could also be used in the standard of care group for established indications like following large volume paracentesis, spontaneous bacterial peritonitis, or hepatorenal syndrome. The median dose of albumin given in the standard of care group was 20g compared with 200g in the albumin group, and ~49.4% of patients in the standard of care group did not receive albumin.
Results
One of the co-primary outcomes was the incidence of new infections as defined by diagnosis by the attending physician (validated by a blinded panel), which occurred in 79 patients of the daily albumin infusion group and 71 in the standard of care group (20.8% vs. 17.9%, p <0.87), which was not statistically significant.
Another co-primary outcome was kidney dysfunction defined by serum creatinine that was 50% or higher, serum creatinine increase of 0.3 mg/dL or more within 48 hours, or initiation of renal replacement therapy. This occurred in 40 patients of the daily albumin group vs. 57 in the standard of care group (10.5% vs. 14.4 %, p<0.87) and was also not statistically different.
The third co-primary outcome was death between day 3 to day 15. There was no difference noted, with death day 3-15 occurring in 30 patients of the daily albumin group and 33 of the standard of care group (7.9% vs. 8.3%, p <0.87).
Secondary outcomes included deaths on day 28, 3 months, and 6 months which were also not significant. There were more occurrences of lung infection in patients receiving daily albumin infusions compared to standard of care (15 vs 8) and more pulmonary edema (15 vs. 4).
Discussion
The ATTIRE trial failed to demonstrate a benefit of targeted albumin therapy over standard of care in preventing new infections, kidney dysfunction, or death in patients with decompensated cirrhosis. There was also no significant benefit in the secondary outcomes of incidences of death at 28 days, 3 months, and 6 months.
There are some limitations of this study that are worth mentioning. Firstly, the trial was not blinded, though this would tend to favor the intervention. Also, the population was not extremely ill, with an average MELD score of 19. Whether targeted albumin would still benefit patients with a higher MELD and perhaps higher risk of infection and kidney injury, is unclear.
The data from this study contrasts prior studies that showed albumin decreased systemic inflammation. Albumin continues to have indications in patients with decompensated cirrhosis to helping counter peripheral vasodilation, after large volume paracentesis, and in patients with spontaneous bacterial peritonitis and hepatorenal syndrome. It is important to remember that albumin infusions carry some risk, with ATTIRE showing more fluid overload and pulmonary edema and more lung infections. Albumin infusions are frequently used as part of treatment plans of patients with decompensated cirrhosis and the amount given needs to be monitored closely to ensure more benefits than risks.
| F: Follow up | 3 years |
| R: Randomization | Therapy |
| I: Intention to treat | Yes |
| S: Similar at baseline | patients with decompensated cirrhosis mostly caused by ETOH abuse with similar MELD scores, Cr, and serum albumin levels |
| B: Blinding | NO |
| E: Equal treatment | Yes |
| S: Source (funding) | Health Innovation Challenge Fund, consulting fees from Allergan, AstraZeneca, Grifols Biologicals, Ipsen Biopharmaceuticals, and Novo Nordisk |
- Bajaj JS, O’Leary JG, Reddy KR, et al. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) experience. Hepatology 2012;56:2328-2335.
- Fernández J, Clària J, Amorós A, et al. Effects of albumin treatment on systemic and portal hemodynamics and systemic inflammation in patients with decompensated cirrhosis. Gastroenterology 2019;157:149-162.
- Fernández J, Angeli P, Trebicka J, et al. Efficacy of albumin treatment for patients with cirrhosis and infections unrelated to spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol 2020;18(4):963-973.e14.